Chapter 11 URODYNAMICS
The term urodynamics was first coined by Davis1 in 1953 to define the study of the storage and emptying phases of the lower urinary tract. Patients with voiding and storage symptoms cannot be reliably diagnosed by history and physical examination alone.2,3 Urodynamic studies offer objective measurements of bladder and urethral functions and dysfunctions while reproducing the patient’s presenting symptoms.
REPRODUCTION OF SYMPTOMS FOR URODYNAMIC EVALUATION
The urodynamic armamentarium is extensive, including bedside eyeball urodynamics, noninvasive uroflowmetry, and multichannel fluoroscopic studies (Table 11-1). A “reflex hammer” approach to urodynamic testing is condemned. Before any urodynamic evaluation, the clinician must formulate specific questions about the case, and a working diagnosis must be in place. The most accurate and least invasive study tailored to answer specific questions and to confirm the diagnosis is performed. It is crucial that urodynamic tests reproduce the patient’s presenting symptoms. A study that does not duplicate the patient’s symptoms is not diagnostic.4 For instance, if a patient states that she loses urine only in an upright position, little is gained by a supine cystometrogram.5 Failure to record an abnormality on urodynamic assessment does not rule out its clinical existence.4 Conversely, not all abnormalities detected on urodynamic tests are clinically significant.4 If urodynamic testing reveals information that is totally unexpected, the history and working diagnosis should be re-evaluated.
Table 11-1 The Urodynamic Armamentarium
| Phase | Study of Bladder Functions | Study of Urethral Functions |
|---|---|---|
| Storage | ||
| Voiding |
INDICATIONS, CONTRAINDICATIONS, AND PATIENT PREPARATION
Urodynamic assessment is indicated if the diagnosis is uncertain, empirical treatment has failed, or an invasive procedure or surgery is contemplated. Urodynamic testing is deferred during an active urinary tract infection or after recent instrumentation. When possible, patients with a chronically indwelling catheter should be started on intermittent catheterization before the study because bladder sensation, capacity, and compliance may be altered by a chronic Foley catheter. Routine antibiotic prophylaxis is unnecessary unless the patient is at high risk for urinary infection, endocarditis, or prosthetic infection.6 Patients with a history of or at risk for autonomic dysreflexia (i.e., T6 or above spinal cord injury) should be pretreated with oral nifedipine or α-blockers and have their blood pressures monitored during urodynamic studies.7,8 If sweating, headache, flushing, severe hypertension, and reflex bradycardia do not respond to bladder drainage, oral nifedipine or intravenous hydralazine, or both, should be administrated immediately. Pharmacologic agents may alter bladder and sphincter functions. Whether these medications should be stopped before the study depends on the goal of the study. If the goal is to evaluate the response to medications (e.g., response of bladder compliance to anticholinergics), the medications should be taken. If the goal is to uncover the cause of urge symptoms, the medications should be stopped before the study.
Urodynamic Evaluation for Stress Urinary Incontinence
Classically, preoperative urodynamic assessments help to define the exact cause of incontinence and therefore guide the SUI surgical approach; evaluate detrusor function (e.g., capacity, instability, poor contractility) and identify patients at risk for voiding dysfunction (i.e., instability, retention) after SUI surgery; predict the impact of prolapse and its correction on storage and voiding functions; and identify urodynamic factors (e.g., high detrusor leak point pressure) that place the upper tract at risk postoperatively.9 In the modern era of minimally invasive pubovaginal and mid-urethral slings, the roles of preoperative urodynamics become more controversial. Although few would argue that additional information could be gleaned from preoperative testing (albeit with a finite risk of urinary infection), it remains unclear whether urodynamics can improve SUI surgical success or alter the surgical approach.10,11 Pubovaginal and mid-urethral slings appear to have reasonable success for any type and severity of SUI.12–15 Patients without preoperative urodynamic evaluation before mid-urethral synthetic slings appear to do as well as those who underwent preoperative urodynamics routinely.16 Nevertheless, urodynamics may identify subpopulation of patients at risk for postoperative failure or voiding complications (e.g., urge, retention).17–19
EVALUATION OF STORAGE FUNCTION
Cystometrography
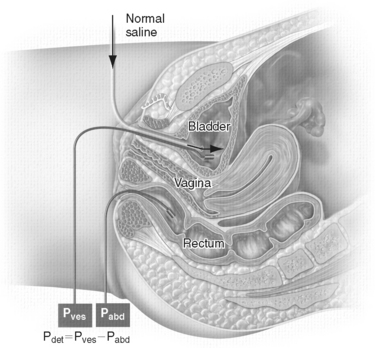
A cystometrogram measures the intravesical pressure (Pves) during bladder filling. The bladder is filled physiologically (i.e., diuresis) or through a catheter using room-temperature saline, water, or contrast (for video urodynamic studies [VUDS]). Fluid infusion is preferred over gas (CO2) infusion because the fluid is less irritative to the bladder than CO2, fluid is noncompressible and can detect smaller detrusor contractions than CO2, fluid leakage (i.e., incontinence) can be easily demonstrated, and leak point pressures, pressure-flow studies (PFSs), and anatomic studies can be performed using fluid but not a gaseous medium. Pressure is transmitted through an intravenous line to an external strain gauge transducer, or it is measured directly on a catheter-mounted, solid-state microtip transducer or fiberoptic transducer.20
In the single-channel cystometrogram, only Pves is monitored, whereas in the multichannel cystometrogram, the Pves and intra-abdominal pressure (Pabd) are measured. A rectal balloon catheter is advanced well past the anal sphincter to measure Pabd to avoid interference with rectal contractions.21 In patients with no anus (e.g., after abdominoperineal resection), Pabd can be monitored inside a colostomy, ileostomy, or vagina. Detrusor pressure (Pdet) is calculated by subtracting intra-abdominal pressure from intravesical pressure (Pdet = Pves − Pabd) (Fig. 11-1). Pdet is a computer-generated number and is subject to error if negative abdominal pressures are recorded. Having both Pves and Pabd monitored simultaneously allows the examiner to differentiate bladder contractions from abdominal straining. This is particularly useful in the evaluation of SUI to differentiate stress-induced detrusor overactivity from genuine SUI during cystometrography; in the evaluation of obstruction to distinguish bladder hypocontraction or straining from high-pressure detrusor contraction during PFSs; and to monitor bladder behavior during leak point pressure determinations and VUDS.
The rate of bladder filling (slow: <10 mL/min; medium: 10 to 100 mL/min; fast: >100 mL/min; physiologic: ≤body weight [kg]/4 [mL/min]; nonphysiologic) and the size of urethral catheter must be specified. Most patients are filled at medium rate initially. Filling is slowed if poor compliance, neurogenic bladder, decreased capacity, or excessive detrusor overactivity is encountered. Filling is increased during provocative maneuvers. Because large catheters may cause obstruction, smaller catheters (<10 Fr) are used.22 A two-catheter technique, in which the larger, infusing catheter (8 to 12 Fr) is removed before voiding (i.e., voids along the smaller, pressure-monitoring catheter [3 to 4 Fr), has been described.23
To avoid artifacts, all lines are flushed to remove any air, and all transducers are zeroed at the superior edge of the pubic symphysis.25 The patient is then asked to perform a Valsalva maneuver to ensure equal pressure transmission to the Pves and Pabd catheters. Changes in Pabd must be accompanied by corresponding changes in Pves; otherwise, the calculated ΔPdet represents artifacts rather than true detrusor contractions. Potential source of errors include pressure measurement artifacts (e.g., air bubbles and kinks in tubing, incorrect placement or migration of catheters, rectal contractions, incorrect zeroing), infusion artifacts (e.g., too rapid infusion in neurogenic and overactive bladders), unanticipated “pop-off” mechanisms (e.g., incompetent urethra that leaks, large bladder diverticulum or vesicoureteral reflux that falsely improves bladder compliance), and patient-related issues (e.g., lack of cooperation, ineffective communication, anxiety, psychological inhibition).21,24
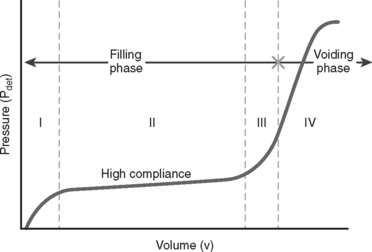
Parameters that are measured by cystometrography include the following:
Detrusor Leak Point Pressure
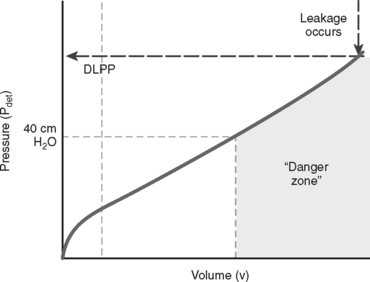
A concept first introduced by McGuire and associates31 in 1981 in the evaluation of myelodysplasia patients, detrusor leak point pressure (DLPP) is defined as the lowest detrusor pressure (Pdet) at which leakage occurs in the absence of detrusor contraction or increased abdominal pressure (Fig. 11-3).25 The bladder is filled until overflow incontinence occurs, and the instantaneous Pdet at which leakage occurs (i.e., DLPP) reflects the resistance of the urethra against the expulsive force of bladder storage pressure. When outlet resistance is high, high bladder pressure is needed to overcome this resistance and cause leakage. Bladder pressure higher than 40 cm H2O impedes ureteral peristalsis, causes hydroureters, and damages the upper tracts. In the classic study of McGuire and colleagues,31 81% and 68% of myelodysplasia patients with DLPP greater than 40 cm H2O developed hydronephrosis and vesicoureteral reflux, respectively. In long-term follow-up, 100% of patients with DLPP greater than 40 cm H2O exhibited upper tract deterioration or reflux, or both.32 A DLPP higher than 40 cm H2O is a prognostic marker for upper tract damage.
Patients with low bladder compliance and a low DLPP may be floridly incontinent, but their upper tracts are safe because the low-resistance urethra functions as a pop-off mechanism to relieve the high detrusor pressure. Patients with low bladder compliance and a DLPP higher than 40 cm H2O risk upper tract damage unless the outlet resistance is reduced or compliance is improved with medication or surgery. Correction of outlet resistance in patients with detrusor–external sphincter dyssynergia (DESD) by sphincter dilation results in an immediate decrease in DLPP and a gradual but significant improvement of bladder compliance over time.33 Failure to reduce DLPP to below 40 cm H2O after sphincterotomy predicts surgical failure, persistent DESD, and upper tract deterioration.34 The use of intermittent catheterization, anticholinergics, and vesicostomy are effective in protecting the upper tracts of neonates with myelodysplasia.35 Plotting the danger zone on a filling cystometrogram is an effective method to establish a storage baseline for patients with neurogenic dysfunction and subsequently track effective management by reducing the danger zone.
Abdominal Leak Point Pressure
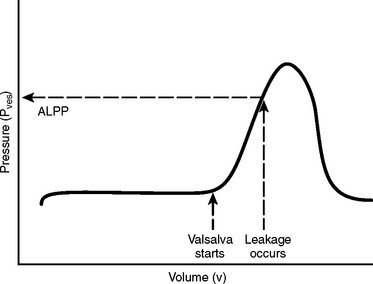
The idea of ALPP emerged from McGuire’s group a decade after the description of DLPP.36 Originally designed to categorize women with SUI into two groups—urethral hypermobility and intrinsic sphincter deficiency (ISD)—ALPP measurement and Q-tip examination became indispensable tools in the diagnosis of SUI. The International Continence Society defined ALPP as the intravesical pressure (Pves) at which urine leakage occurs due to increased abdominal pressure in the absence of a detrusor contraction.25 The bladder is half-filled to an arbitrary volume of 200 to 250 mL. The patient is then asked to perform a Valsalva maneuver or cough until leakage occurs. If no leakage is observed, the bladder is filled in 50-mL increments. The smallest recorded Pves associated with urodynamic demonstration of SUI is the ALPP (Fig. 11-4). ALPP (leakage) usually occurs on the upward slope of the curve and not at the peak pressure generated unless the peak pressure represents the exact ALPP (i.e., exact moment of incontinence).
Abdominal Leak Point Pressure versus Detrusor Leak Point Pressure
Unlike DLPP, which is a static reflection of urethral resistance to bladder intrinsic storage pressure, ALPP measures the dynamic urethral resistance to brief increases in abdominal pressure. Abdominal pressure (ALPP) and detrusor pressure (DLPP) are different expulsive forces with respect to the urethra. Whereas detrusor pressure tends to open the bladder neck, abdominal pressure tends to close the internal sphincter shut. Normally, the internal sphincter does not leak or open, regardless of how much abdominal pressure is exerted. For instance, during blunt trauma to a full bladder, the bladder will rupture before the bladder neck is forced open. If SUI occurs as a result of an increase in abdominal pressure, the proximal urethra and bladder neck are rotated and descended away from its resting intra-abdominal position during a Valsalva maneuver (i.e., urethral hypermobility), or there is an intrinsic malfunction of the internal urethral sphincter (i.e., ISD).37 All women with urethral hypermobility and SUI are considered to have some degree of ISD, because the normal internal sphincter should remain closed no matter how much stress and rotational descent it experiences.23 ISD is a spectrum of urethral dysfunction.
Abdominal Leak Point Pressure, Urethral Hypermobility, and Internal Urethral Sphincter
SUI patients with urethral hypermobility leak at considerably higher abdominal pressures than those with pure ISD. Leakage at an ALPP less than 60 cm H2O is characteristic of ISD. Eighty-one percent of patients with an ALPP less than 60 cm H2O reported a history of severe incontinence, and 76% of patients with an ALPP less than 60 cm H2O demonstrated type III SUI on fluoroscopic studies (i.e., no urethral hypermobility). Leakage at an ALPP greater than 90 cm H2O is indicative of urethral hypermobility. These patients reported lesser degrees of incontinence and exhibited type I or type II SUI on VUDS (i.e., minimal to gross hypermobility).36 Patients with an ALPP between 60 and 90 cm H2O have type II or type III SUI. Patients with an ALPP less than 60 cm H2O classically failed to respond to suspension operations designed for the hypermobile urethra. They should be treated with pubovaginal slings, periurethral bulking agents (if there is no associated hypermobility), or artificial sphincters. Subdividing patients into hypermobility or ISD groups based on ALPP measurement and Q-tip test results on physical examination may become less important because pubovaginal slings and mid-urethral slings have been shown to be effective for anatomic incontinence.12–15
Abdominal Leak Point Pressure and Pelvic Organ Prolapse
ALPP measurements are more difficult to interpret in the presence of pelvic organ prolapse. Anterior vaginal wall prolapse may falsely elevate the ALPP because the prolapse functions as a sink to dissipate and absorb the effect of abdominal pressure on the proximal urethra.38 The urethra may be kinked or compressed by the prolapsed organ, causing partial obstruction and elevating the ALPP. This is why patients with high-grade cystoceles rarely complain of clinical SUI. When the prolapse is reduced, up to 60% of patients with grade 1 to 2 cystocele and 91% of patients with grade 3 to 4 cystocele who do not complain of incontinence demonstrate SUI on urodynamic evaluations.39 If the cystocele is repaired without addressing the urethra, occult stress incontinence may be unmasked postoperatively. It is unclear what percentage of patients with no symptoms of SUI will be symptomatic after a prolapse repair. It is also unclear whether performing ALPP with a pessary helps to predict that population. Whether prophylactic sling should be placed at the time of concomitant prolapse surgery and what roles preoperative ALPP plays in that decision remain controversial. Nevertheless, all patients undergoing ALPP measurements should have a pelvic examination in supine and upright positions to determine whether prolapse exists. If significant prolapse is found, upright ALPP measurements should be repeated with the prolapse reduced.40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree