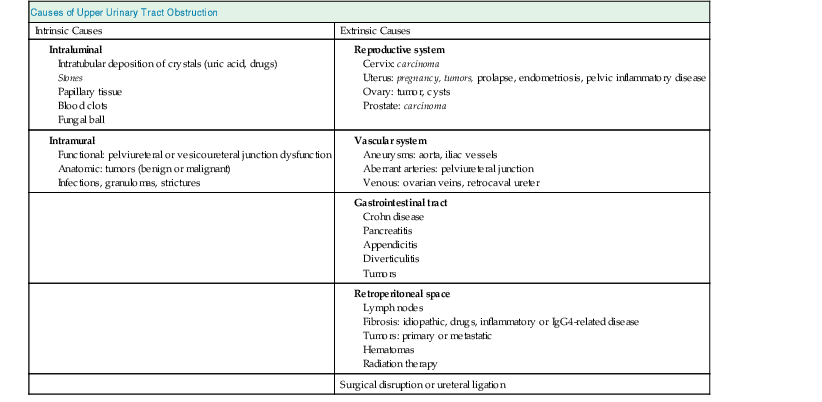
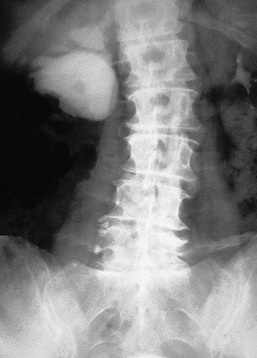
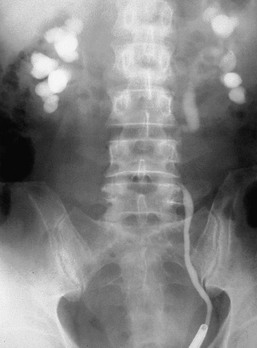
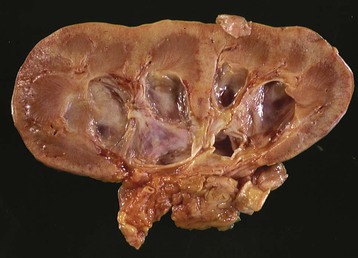
Jeremy Hughes Obstructive uropathy refers to the structural or functional changes in the urinary tract that impede normal urine flow. Obstructive nephropathy refers to the renal disease caused by impaired flow of urine or tubular fluid. Hydronephrosis refers to dilation of the urinary tract. Hydronephrosis is not synonymous with obstructive uropathy because the former can occur without functional obstruction to the urinary tract and can be absent in established obstruction. Obstructive uropathy and nephropathy frequently coexist, and their management requires close collaboration between nephrologists and urologists. Some surgical aspects of obstruction to the urinary tract are discussed in Chapter 61. Obstructive uropathy is classified according to the site, degree, and duration of the obstruction. Acute or chronic obstruction can occur anywhere in the urinary tract and includes intrarenal causes (casts, crystals) and extrarenal causes. Acute or chronic obstruction is further subdivided into upper urinary tract obstruction (usually unilateral obstruction occurring above the vesicoureteral junction) and lower urinary tract obstruction (usually bilateral obstruction located below the vesicoureteral junction). Complete obstruction of the urinary tract is termed high grade, whereas partial or incomplete obstruction is termed low grade. Unilateral obstruction in a patient with two normal kidneys will not result in significant renal impairment because the contralateral kidney compensates. However, bilateral obstruction or the obstruction of a single functioning kidney will result in renal failure. In acute urinary tract obstruction, changes are mainly functional, whereas structural damage to the kidney results from more chronic obstruction. The kidney with acute functional changes may recover after the effective release of the obstruction, but structural changes may be permanent and lead to chronic renal impairment. Urinary tract obstruction remains a major cause of renal impairment worldwide in children and adults. The causes of obstructive uropathy affecting the upper and lower urinary tracts are summarized in Table 60-1 and Box 60-1. Congenital urinary tract obstruction occurs most frequently in males, most commonly as a result of either posterior urethral valves or pelviureteral junction (PUJ) obstruction. If obstruction occurs early during development, the kidney fails to develop and becomes dysplastic. If the obstruction is bilateral, there is a high mortality rate as a result of severe renal failure. If the obstruction occurs later in gestation and is low grade or unilateral, hydronephrosis and nephron loss will still occur, but renal function may be sufficient to allow survival, and such patients may not present until later in childhood. Pelviureteral junction obstruction, if it is mild, may not present until adulthood and in some patients may be an incidental finding (Fig. 60-1). However, with increased use and improved sensitivity of antenatal scanning, congenital abnormalities of the urinary tract are now frequently identified early, allowing prompt postnatal (and in some cases antenatal) intervention to relieve the obstruction and hence to preserve renal function. Congenital causes of obstruction are discussed further in Chapter 52. Acquired urinary tract obstruction may affect either the upper or lower urinary tract and can result from either intrinsic or extrinsic causes. Intrinsic causes of obstruction may be intraluminal or intramural. Intraluminal obstruction may result from tubular intrarenal obstruction, such as the deposition of uric acid crystals in the tubular lumen after treatment for hematologic malignancies (tumor lysis syndrome). It may also occur with the precipitation of Bence Jones protein in myeloma and with the precipitation or crystal formation of a number of drugs, including sulfonamides, acyclovir, methotrexate, and indinavir. Uncommonly, patients with an underlying glomerulonephritis such as IgA nephropathy may develop severe glomerular hematuria with tubular obstruction from erythrocytes and acute renal dysfunction that typically resolves with time. Extrarenal intraluminal obstruction in young adults is most commonly caused by renal calculi (see Chapter 59). Calcium oxalate stones are the most common and typically cause intermittent acute unilateral urinary tract obstruction but rarely result in marked chronic renal impairment. Less common causes of urinary lithiasis, such as struvite stones, uric acid stones, and cystinuria are often bilateral and hence more likely to cause long-term renal impairment. Renal calculi lodge more commonly in the calyx, PUJ, or vesicoureteral junction and at the level of the pelvic brim. Surgical management of stones is discussed in Chapter 61. Intraluminal obstruction can also result from a sloughed papilla after papillary necrosis, or blood clots after macroscopic hematuria (clot colic). Papillary necrosis may occur in diabetes mellitus, sickle cell trait or disease, analgesic nephropathy, renal amyloidosis, and acute pyelonephritis. Clot colic can occur with bleeding from renal tumors or arteriovenous malformations, after renal trauma, and in patients with polycystic kidney disease. Intramural obstruction can result from either functional or anatomic changes. Functional disorders include adynamic ureteral segments (usually at the junction of the ureter with the pelvis or bladder) and neurologic disorders. The latter may result in a contracted (hypertonic) bladder or a flaccid (atonic) bladder, depending on whether the lesion affects upper or lower motor neurons, and may lead to impaired bladder emptying with vesicoureteral reflux. Bladder dysfunction is very common in patients with multiple sclerosis and after spinal cord injury and is also seen in diabetes mellitus, in Parkinson disease, and after cerebrovascular accidents. Some drugs (anticholinergics, levodopa) can alter neuromuscular activity of the bladder and result in functional obstruction, especially if there is preexisting bladder outflow obstruction (e.g., prostatic hypertrophy). Anatomic causes of intramural obstruction of the upper urinary tract include transitional cell carcinoma of the renal pelvis and ureter and ureteral strictures secondary to radiotherapy or retroperitoneal surgery. Rarely, obstruction may result from ureteral valve malfunction, polyps, or strictures after therapy for tuberculosis. Intramural obstruction of the lower urinary tract can result from urethral strictures, which are usually secondary to chronic instrumentation or previous urethritis, or malignant and benign tumors of the bladder. Infection with Schistosoma haematobium when the ova lodge in the distal ureter and bladder is a common cause of obstructive uropathy worldwide; up to 50% of chronically infected patients develop ureteral strictures and fibrosis, with contraction of the bladder. The most common cause of extrinsic compression in women is pressure from a gravid uterus on the pelvic rim; the right ureter is more commonly affected. It is usually asymptomatic, and the changes resolve rapidly after delivery. Rarely, bilateral obstruction and acute kidney injury (AKI) may occur. Ureteral dilation may frequently be seen in pregnancy as a result of hormonal effects (especially progesterone) on smooth muscle, but this does not indicate functional obstruction (see Chapter 43, Fig. 43-1). Carcinoma of the cervix may also cause extrinsic obstruction secondary to direct extension of the tumor to involve the urinary tract. Other pelvic pathologic processes that can cause ureteral compression include benign and malignant uterine and ovarian masses, abscesses, endometriosis, and pelvic inflammatory disease. Compression of the ureters outside the bladder may also occur with uterine prolapse. Rarely, inadvertent ureteric ligation may occur during surgical procedures, particularly those related to obstetrics and gynecology. Unilateral ligation may go undetected, but AKI will result from bilateral ligation. In men, the most common cause of extrinsic obstruction of the lower urinary tract is benign prostatic hypertrophy. Carcinoma of the prostate can also result in obstruction either from direct tumor extension to the bladder outlet or ureters or from metastatic spread. Retroperitoneal pathology may also result in extrinsic obstruction of the ureters, as can metastases or extension of tumors from the cervix, prostate, bladder, colon, ovary, and uterus. Primary tumors of the retroperitoneum, such as lymphomas and sarcomas, can commonly cause obstruction. Obstruction can also result from inflammatory conditions affecting the retroperitoneum, such as Crohn disease and large bowel diverticulitis. In Crohn disease the obstruction is usually right sided as a result of ileocecal disease. Less common pathologic processes include retroperitoneal fibrosis, in which thick fibrous tissue extends out from the aorta to encase the ureters and draw them medially (Fig. 60-2). Retroperitoneal fibrosis may be idiopathic but can result from inflammatory aortic aneurysms, certain drugs (e.g., β-blockers, bromocriptine, and methysergide), previous irradiation, trauma or surgery, and granulomatous disease (e.g., tuberculosis, sarcoidosis). Retroperitoneal fibrosis is also associated with IgG4-related disease, which typically presents with autoimmune pancreatitis, and elevated serum IgG4 levels suggests this diagnosis.1 IgG4-related disease is also suggested in retroperitoneal biopsy material by an IgG4 positive plasma cell infiltrate, fibrosis with a whorled cartwheel pattern, and an obliterative venous phlebitis.2 Ureteral compression may also be a result of vascular abnormalities, including aneurysmal dilation of the aorta or iliac vessels, aberrant vessels, and anatomic variations in the location of the ureter (retrocaval ureter). Obstruction of the renal tract causes profound functional and structural changes of the kidney. Initially, changes are predominantly functional and potentially reversible, but with time, chronic and irreversible structural changes occur. Our understanding of the consequences of urinary tract obstruction stems mainly from the study of animal models.3 Although many studies have focused on the effects of complete ureteral obstruction in rodents, investigators have also examined models of chronic complete, partial, or reversible obstruction in adult and neonatal animals.3 Available experimental data show little species-to-species variation in the response to acute obstruction, suggesting that similar changes are likely to occur in humans. The complex effects of urinary tract obstruction on the kidney affect both glomerular hemodynamics and tubular function. Glomerular filtration rate (GFR) declines progressively after the onset of complete ureteral obstruction.4 Glomerular filtration is determined by the mean hydraulic pressure gradient between the glomerular capillary lumen and Bowman space, the renal plasma flow, the ultrafiltration coefficient of the glomerular capillary wall, and the mean oncotic pressure difference across the glomerular wall. Obstruction can affect all of these, and the effects vary with the duration of the obstruction, the hydration state, and presence or absence of a contralateral functioning kidney. After complete ureteral obstruction, there is an initial rise in proximal tubular pressure. At the same time, afferent arteriolar dilation occurs as a result of the generation of vasodilatory prostaglandins. Glomerular capillary hydraulic pressure increases, but this does not offset the rise in tubular pressure, and there is a net decrease in the hydraulic pressure gradient across glomerular capillaries, resulting in an 80% decline in GFR.4 About 2 to 5 hours after obstruction, renal blood flow begins to decline, whereas intratubular pressure continues to increase. Within 5 hours, proximal tubular pressure begins to decline toward control values. From this time, the main determinant of the decrease in GFR is the fall in intraglomerular capillary pressure as a result of an increase in resistance of afferent arterioles. This results in a progressive fall in renal plasma flow, which reaches 30% to 50% of control values by 24 hours. Preferential constriction of the preglomerular blood vessels lowers both plasma flow and glomerular capillary pressure, resulting in a greater decrement in GFR than in plasma flow and a fall in filtration fraction. A falling filtration fraction also results from diversion of blood to nonfiltering areas of the kidney or a reduction in the ultrafiltration coefficient.4 The relative changes in ureteral pressure, renal plasma flow, and GFR are summarized in Figure 60-3. The intrarenal vasoconstriction results from the generation of angiotensin II and thromboxane A2, the release of vasopressin (antidiuretic hormone), and decreased nitric oxide production. Intrarenal angiotensin II generation occurs secondary to an increase in renin release either through reduced delivery of sodium and chloride to the distal nephron (macula densa mechanism) or through a reduction in transmural pressure at the baroreceptor as a consequence of the prostaglandin-dependent dilation of the afferent arteriole. Generation of thromboxane A2 occurs in both glomeruli and infiltrating interstitial cells. Angiotensin II and thromboxane A2 may also reduce the ultrafiltration coefficient. The central role of these two vasoconstrictors has been demonstrated by studies in rats, in which pretreatment with angiotensin-converting enzyme inhibitors and thromboxane synthase inhibitors virtually normalized renal function after the relief of short-term ureteral obstruction.5 An interstitial leukocyte infiltrate, predominantly macrophages, develops in response to chemoattractants such as monocyte chemoattractant protein 1 and osteopontin expressed by tubular cells. This infiltrate plays a key role in the acute functional changes after ureteral obstruction6 and the pathogenesis of the late structural changes that occur after obstruction as macrophage depletion limits experimental interstitial fibrosis.7 The extent to which glomerular function recovers after the release of ureteral obstruction depends on the duration of the obstruction. Whole kidney GFR may return to normal after short-term obstruction (days), whereas recovery may be incomplete after prolonged obstruction. Evidence from studies in rats suggests that even with shorter periods of obstruction (72 hours), there may be a permanent loss of nephrons, and whole kidney GFR returns to normal only at the expense of hyperfiltration (increase in single nephron GFR) in the remaining functional nephrons.8 Abnormalities in tubular function are common in urinary tract obstruction and manifest as altered renal handling of electrolytes and changes in the regulation of water excretion.9 The degree and nature of the tubular defects after obstruction depend in part on whether the obstruction is bilateral or unilateral. These differences could result from the dissimilar hemodynamic responses, different intrinsic changes within the nephron, differences in extrinsic factors (e.g., volume expansion and accumulation of natriuretic substances in bilateral obstruction) between the two states, or a combination of all three. After ureteral obstruction, the ability to concentrate the urine is markedly impaired, with maximum values of 350 to 400 mOsm/kg reported in the rat. Causative factors include a loss of medullary tonicity, an overall decrease in GFR in deep nephrons, and a reduced expression of sodium transporters.10 Also, the collecting duct is unresponsive to vasopressin because of a reduction in the expression of renal aquaporins that results from both cyclooxygenase-2 activity11 and angiotensin II.12 Rats exhibit reduced expression of multiple acid-base transporters after ureteral obstruction,13 and patients with urinary tract obstruction often exhibit urinary acidification defects. These defects may be detected only by exogenous acid loading, but hyperchloremic acidosis caused by impaired distal acid secretion, hyporeninemic hypoaldosteronism (type IV renal tubular acidosis), and a combination of these findings have been described. This acidifying defect results from a marked increase in bicarbonate excretion or from a distal acidification defect, possibly as a result of abnormalities of the H+-ATPase activity of intercalated cells of the collecting duct after ureteral obstruction. Obstruction alters renal potassium handling. In the presence of a normal functioning contralateral kidney, potassium excretion is reduced after relief of obstruction, either in proportion to or perhaps even exceeding the fall in GFR (i.e., fractional excretion of potassium is unaltered or slightly reduced). There is a defect in the distal potassium secretory mechanism after unilateral obstruction that may be secondary to reduced responsiveness of that nephron segment to aldosterone. By contrast, after release of bilateral ureteral obstruction, there is a marked increase in both net and fractional potassium excretion. The major mechanism by which potassium losses occur in this setting is an increased delivery of sodium to the distal tubule, resulting in an accelerated sodium-potassium exchange. Recovery of tubular function after release of obstruction is slow and may remain abnormal even after whole-kidney GFR has returned to normal. In rats, acidification and potassium-handling abnormalities persist for at least 14 days and urinary concentrating ability is abnormal for up to 60 days after the release of 24 hours of unilateral ureteral obstruction. These observations are consistent with persistent alterations in distal tubular and collecting duct function or a loss in functioning juxtaglomerular nephrons after the release of the obstruction. The morphologic alterations in renal architecture are similar irrespective of the cause of the obstruction. Initially, renal enlargement and edema occur with pelvicalyceal dilation (Fig. 60-4). Tubular dilation that predominantly affects the collecting duct and distal tubular segments develops microscopically, although cellular flattening and atrophy of proximal tubular cells can also occur. Glomerular structures are usually preserved initially, although Bowman space may be dilated and some periglomerular fibrosis may ultimately develop.
Urinary Tract Obstruction
Definitions
Etiology and Pathogenesis
Congenital Urinary Tract Obstruction
Acquired Urinary Tract Obstruction
Intrinsic Obstruction
Intraluminal Obstruction
Intramural Obstruction
Extrinsic Obstruction
Pathophysiology
Changes in Glomerular Function
Changes in Tubular Function
Histopathologic Changes