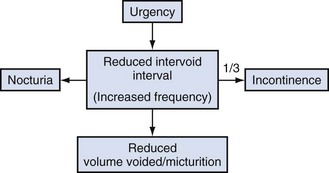
Christopher R. Chapple, MD, FRCS (Urol), FEBU, Ian Milsom, MD, PhD Urinary incontinence (UI) has a considerable social and economic impact. Indeed, it has been estimated that UI in women was the primary cause for more than 1.1 million office visits in 2000 in the United States (Litwin et al, 2005). Hu and colleagues (2004) estimated the total direct and indirect cost of incontinence in the United States in the year 2000 to be approximately $19.5 billion. Incontinence has a larger economic impact than many chronic conditions and diseases. The “storage” component of lower urinary tract symptoms (LUTS), of which incontinence is unarguably the most troublesome, is a common and important cause of morbidity and impairment of quality of life, in both men and women (Chapple et al, 2008). In order to effectively consider the subject of incontinence, it is important to think in terms of symptoms, signs, urodynamic observations, and conditions, as defined by the International Continence Society (ICS) and summarized in their published standardization report (Abrams, 2002; Abrams et al, 2002). Overactive bladder (OAB), or the urgency frequency symptom syndrome, comprises urgency, with or without urgency incontinence, usually with frequency and nocturia. Urgency is widely held to be a key symptom driving the clinical sequence as demonstrated by Figure 63–1. Figure 63–1 The overactive bladder symptom complex. (From Chapple CR, Artibani W, Cardozo LD, et al. The role of urinary urgency and its measurement in the overactive bladder symptom syndrome: current concepts and future prospects. BJU Int 2005;95:335–40.) (From Wein AJ, Rackley RR. Overactive bladder: a better understanding of pathophysiology, diagnosis and management. J Urol 2006;175:S5–10.) More than 40% of women with urethral sphincter incompetence will have a significant cystocele (Cardozo and Stanton, 1980). Conversely, a history of stress incontinence associated with a mild or moderate cystocele is not specific for diagnosing genuine stress incontinence (Summitt et al, 1992). “Occult or latent” incontinence is urethral sphincteric incompetence masked by the presence of pelvic prolapse (Rosenzweig et al, 1992a, 1992b). Not infrequently, incontinent women may note the decrease or disappearance of stress incontinence episodes as the degree of prolapse worsens. The sign of occult stress incontinence is facilitated by the use of a speculum or pessary to reduce the prolapse while a stress maneuver is performed. The method to reduce vaginal prolapse to evaluate latent incontinence is not universally agreed on or standardized at this time. Vaginal examination allows the description of observed and palpable anatomic abnormalities and the assessment of pelvic floor muscle function. Segments of the lower reproductive tract should be considered as a substitute for terms as “cystocoele, rectocoele, enterocoele, or urethrovesical junction” because these terms convey an unrealistic certainty relating to the structures associated with the vaginal bulge, especially following previous prolapse surgery. This section draws heavily on the ICS standardization report, which commented on the evaluation of pelvic organ prolapse (Bump et al, 1996) as modified by the ICS standardization report 2002. Patients with severe prolapse may develop voiding symptoms as a result of urethral kinking, leading to obstruction that is worsened during straining effort (Richardson et al, 1983). For instance, a moderate or severe cystocele may promote urethral compression and kinking, pressure dissipation, and an increase in maximum urethral closure pressures (Bergman et al, 1988; Versi et al, 1998). Clearly, a number of storage urinary symptoms may occur in combination with prolapse. Risk factors contributing to the symptoms of urgency and frequency include age and urogenital atrophy. One study found that women with mild cystoceles had a 20% incidence of detrusor overactivity, and the incidence increased to 52% in those with moderate to severe cystoceles (Enhorning, 1961). More than 40% of women with urethral sphincter incompetence will have a significant cystocele (Cardozo and Stanton, 1980). A complaint of stress incontinence associated with the appearance of a mild or moderate cystocele, however, is not specific for diagnosing genuine stress incontinence (Walters and Shields, 1988; Summitt et al, 1992). Occult or latent incontinence is urethral sphincteric incompetence masked by the presence of pelvic prolapse (Rosenzweig et al, 1992). Not infrequently, incontinent women may note the decrease or disappearance of stress incontinence episodes as the degree of prolapse worsens. The office demonstration of the sign of occult stress incontinence is facilitated by the use of a speculum or pessary to reduce the prolapse while a stress maneuver is performed. Its presence may influence management options given to the patient. However, the method to reduce vaginal prolapse to evaluate latent incontinence is not universally agreed on or standardized at this time. The prevalence of fecal incontinence increases to 17% in populations with pelvic organ prolapse and UI (Jackson et al, 1997) compared with 2% to 3% in the general population (Leigh and Turnberg, 1982; Thomas et al, 1984). The most common mechanisms are an incompetent sphincteric mechanism (secondary to a structural defect or pudendal nerve damage) and overflow incontinence. Sexual function consists of a complex interaction of biologic, psychologic, and social factors that affect both patient and partner. Various studies have described the presence of sexual problems in patients who present with pelvic organ prolapse and UI (Haase and Skibsted, 1988; Field and Hilton, 1993). Key Points: Conditions Associated with Pelvic Organ Prolapse To understand the pathophysiology of urinary incontinence, it is important to be familiar with the micturition cycle and the physiology of normal storage and emptying of urine. The details of the physiology of micturition are provided in Chapter 60. The function of the lower urinary tract is to temporarily store a continuously increasing amount of urine at low pressure and expel it under appropriate circumstances. This is dependent on the coordinated activity of smooth and striated muscles in the bladder, urethra, and pelvic floor. The bladder and urethra constitute a functional unit, which is controlled by a complex interplay between the central and peripheral nervous systems and local regulatory factors (Andersson and Wein, 2004). During bladder filling at physiologic rates, detrusor pressure remains nearly constant because of this property of accommodation (Klevmark, 1974), otherwise known as “tonus” or receptive relaxation. Accommodation accounts for the nearly flat cystometric curve that is seen during normal bladder filling. The viscoelastic properties of the bladder, based on its composition of smooth muscle, collagen, and elastin, normally produce a highly compliant structure. When accommodation is impaired, low bladder compliance ensues. This is manifest as a steep rise in detrusor pressure during bladder filling. In addition to the viscoelastic properties of the bladder, the neural control of the lower urinary tract and the anatomy and support of the sphincteric unit are important factors in the maintenance of urinary continence. The micturition reflex is normally under voluntary control and is organized in the rostral brainstem (the pontine micturition center [PMC]). Micturition results from a release from the negative inhibitor effect of the higher centers on the PMC. It requires integration and modulation by the parasympathetic and somatic components of the sacral spinal cord (the sacral micturition center) and the thoracolumbar sympathetic components (Fowler et al, 2008). The reservoir function of the bladder requires a permanently low intravesical pressure over a wide volume range and a bladder outlet that remains firmly closed, even under conditions of abdominal pressure rises, during the filling phase of the bladder, which represents the majority of its activity cycle. Conversely, during voiding the bladder should be able to develop a sustained contraction of sufficient strength with opening of the bladder outlet offering a low resistance to urinary flow to assure adequate emptying of the bladder. During micturition, the first recorded event is sudden and complete relaxation of the striated sphincteric muscles, characterized by complete electrical silence of the sphincter electromyogram (Tanagho and Miller, 1970). This is followed almost immediately by a rise in detrusor pressure and concomitant fall in urethral pressure as the bladder and proximal urethra become isobaric (Yalla et al, 1980). The bladder neck and urethra open, and voiding ensues. Voluntary interruption of the stream is accomplished by a sudden contraction of the striated periurethral musculature, which, through a reflex mechanism, shuts off the detrusor contraction, aborting micturition. The sphincteric system is also designed to resist physiologic increases in abdominal pressure and thereby prevent the development of incontinence. Normal storage of urine is therefore dependent on the following: Disordered lower urinary tract function can result from the following: Detrusor overactivity is a urodynamic observation characterized by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. Detrusor overactivity may be phasic, terminal, or both (Abrams et al, 2002). There are certain patterns of detrusor overactivity: Detrusor overactivity may also be qualified, when possible, according to cause. Examples follow: In clinical and research practice, the extent of neurologic examination/investigation varies. It is likely that the proportion of neurogenic-to-idiopathic detrusor overactivity will increase if a more complete neurologic assessment is carried out (Table 63–1). Table 63–1 Causes of Detrusor Overactivity High-pressure bladders with detrusor–sphincter dyssynergia are prone to develop vesicoureteral reflux, which may ultimately lead to renal impairment. This can be clearly demonstrated by videocystometrography (VCMG). Preservation of renal function is of utmost importance in the management of patients who have chronic neurologic conditions. As a rule of thumb, those patients with a competent bladder outflow and an end filling pressure of 40 cm H2O or higher before leakage occurs—(the detrusor leak point pressure) are at particular risk of developing upper urinary tract problems due to back pressure (McGuire et al, 1996). Neurologic conditions can alter vesicourethral function by impairing the following: Idiopathic detrusor overactivity can be associated with a variety of non-neurogenic clinical conditions such as bladder outlet obstruction, dysfunctional voiding, and inflammatory reactions (Hebjorn et al, 1976; Awad and McGinnis, 1983; Blaivas, 1988; Resnick et al, 1989; Fantl et al, 1990; Brading and Turner, 1994; Carlson et al, 2001). Elbadawi and colleagues (1993a, 1993b, 1993c) have proposed a possible explanation for age-related detrusor overactivity based on detailed ultrastructural studies of the bladder. They showed a characteristic structural pattern in bladder biopsy specimens obtained from elderly patients with detrusor overactivity. Electron microscopic changes (abundant, distinctive protrusion junctions and abutments) in patients with detrusor overactivity are thought to result in a diminished electrical resistance between detrusor muscle cells and, hence, a hyperexcitable state that results in involuntary detrusor contractions. Bladder outlet obstruction causes increased voiding pressures and a prolonged duration of voiding. It has been hypothesized that this results in ischemic damage to the intramural ganglia and consequently to a partial denervation of the detrusor muscle (Brading and Turner, 1994). Experiments have shown that denervation of smooth muscle cells may result in supersensitivity to agonists, increased excitability, and increased electrical coupling. These changes may make the detrusor muscle susceptible to the development of involuntary contractions. The detrusor muscle hypertrophy on obstruction does not necessarily play a causative role (Brading and Turner, 1994). A somewhat contrasting and more evidence-based explanation comes from the observation of increased levels of nerve growth factor in the bladders of obstructed rats and humans and increased dimensions of afferent and efferent rat neurons (Steers et al, 1991). In many cases the etiology is unclear—the cause of idiopathic detrusor overactivity is likely to be neurogenic rather than myogenic, particularly in view of the association between detrusor overactivity and aging. An increased number of putative afferent nerves in the bladder wall of female patients has been reported (Smet et al, 1997). Although no effect of intravesical capsaicin on idiopathic detrusor overactivity was found (Fowler, 2002), efficacy was found in 12 patients treated with the more potent analog resiniferatoxin (Cruz, 2002). This suggests the involvement of the afferent C fibers. The role of non-neurogenic factors cannot be excluded because abnormal cell-to-cell communications have been reported in the bladders of patients with idiopathic overactivity (Elbadawi et al, 1993b). Although it is well established that high storage pressures can lead to incontinence and upper tract deterioration (McGuire et al, 1981; Comiter et al, 1997), at the present time there are no standardized or international agreed values to define normal, high, and low compliance. The calculated value of compliance is probably less important than the actual bladder pressure during filling. This is because the compliance value can change depending on the volume over which it is calculated. For this reason, numeric values of compliance are rarely reported. For example, sustained detrusor pressures of 35 to 40 cm H2O or greater during storage, regardless of the bladder volume, can lead to upper tract damage, but similar damage can also occur at lower pressures (McGuire et al, 1981; Churchill et al, 1987). When the detrusor pressure exceeds the pressure capacity of the sphincter to resist that pressure, incontinence results. Low bladder compliance denotes an abnormal volume-pressure relationship in which there is a high incremental rise in detrusor pressure during bladder filling. Low bladder compliance may be caused by changes in the elastic and viscoelastic properties of the bladder, changes in detrusor muscle tone, or combinations of the two. Clinically, low bladder compliance is most commonly seen in a variety of neurologic conditions, especially lower motor neuron lesions such as spina bifida and cauda equina syndrome. Obstructive uropathy, because of its effect on bladder ultrastructure, is known to cause low bladder compliance and has been identified as a urodynamic risk factor. Leng and McGuire (2003) showed that relief of obstruction by transurethral incision or resection of the prostate can improve compliance. The clinical causes of low bladder compliance are listed in Table 63–2. Table 63–2 Causes of Low Bladder Compliance The urethra has two main functions: Traditionally, the urethral sphincter is considered to be composed of two components: the internal sphincter, which represents a direct continuation of the detrusor smooth muscle, and the striated external sphincter (Tanagho, 1992). From a clinical standpoint, the bladder neck–proximal urethra normally functions as a sphincter in both sexes. Anatomically there is a unique mixture of smooth and striated muscle, intracellular matrix, and mucosal components contributing to the functional sphincter. In the male there are two important sphincteric mechanisms: (Hadley et al, 1986). Females are much more likely to suffer from UI due to sphincteric deficiency than males because of the much less powerful sphincteric mechanisms. The bladder neck is a far weaker structure than the male bladder neck and is often incompetent, even in nulliparous young women (Chapple et al, 1989). The bladder neck is poorly defined with the muscle fibers having a mainly longitudinal orientation. Urinary continence usually relies on the integrity of the urethral sphincteric mechanism in females (analogous to the distal sphincter in men). Like the male distal mechanism, it is composed of a longitudinal intrinsic urethral smooth muscle and a larger extrinsic striated muscle component. This sphincter extends throughout the proximal two thirds of the urethra, being most developed in the middle one third of the urethra. Therefore in women the majority of the urethra should be considered to be sphincter active. Damage to the innervation of the urethral sphincter (in particular the pudendal nerve) by obstetric trauma reduces the effectiveness of this mechanism and predisposes to stress UI (Table 63–3). Table 63–3 Comparison of Male and Female Sphincteric Mechanisms* * Indicates why females are much more likely to develop an incompetent urethral mechanism and are prone to urinary incontinence from intrinsic sphincter deficiency. The causes of sphincteric dysfunction are different in men and women. Vaginal delivery can cause partial denervation with consequent reinnervation in most first deliveries (Allen et al, 1990). A growing body of evidence indicates that multiparity; forceps delivery; increased duration of the second stage of labor, partially related to epidural anesthesia; third-degree perineal tear; and high birth weight (>4000 g) are important factors leading to pudendal nerve injury (Snooks et al, 1986; Handa et al, 1996; Brown and Lumley, 1998; Rortveit et al, 2003). Snooks and colleagues (1984) studied the postpartum innervation of the external anal sphincter and found that the external anal sphincter and its innervation might be damaged by vaginal delivery but not by cesarean section. The abnormalities found were most marked in multiparas and correlated most strongly with a prolonged second stage of labor and forceps delivery. Several conditions are associated with increased risk for ISD in women: Sphincter abnormalities in men are caused by trauma or neurologic injury. With prostatectomy, whether for benign disease or with radical prostatectomy, the distal urethral sphincter (particularly the rhabdosphincter) can be damaged by direct injury or injury to the nerve supply or supporting structures (Nitti, 2002). In some cases, there may be preexisting damage to the sphincter that cannot be accurately diagnosed preoperatively (Nitti, 2002). As in women (as noted earlier), radiation and neurologic lesions can cause sphincteric dysfunction. Finally, pelvic trauma or instrumentation that results in trauma to the distal urethral sphincter can result in incontinence, particularly when the PUS is absent or deficient. Postprostatectomy incontinence, like any urinary incontinence, may be caused by bladder dysfunction, sphincter dysfunction, or a combination of both and may be associated with other lower urinary tract symptoms. Urodynamic investigations are helpful to rule out bladder outlet obstruction or significant bladder dysfunction. Urodynamic studies have demonstrated that the sphincter incompetence occurs as the sole cause in more than two thirds of patients. Isolated bladder dysfunction (detrusor overactivity, poor compliance, detrusor underactivity during voiding) is uncommon, occurring in less than 10% (Ficazzola and Nitti, 1998; Groutz et al, 2000). However, sphincter and bladder dysfunction can coexist in at least one third of incontinent patients. Bladder dysfunction may occur de novo after prostatectomy, perhaps induced by bladder denervation; may be caused by outlet obstruction; or may be related to preexisting factors such as age. Impaired detrusor contractility and poor compliance resolved in the majority of patients within 8 months (Giannantoni et al, 2004). Decreased sphincter resistance may be due to tissue scarring in some cases and is reflected by a low urethral compliance; however, this parameter is difficult to measure (Groutz et al, 2000). Scarring may lead to an anastomotic stricture and is clinically suspected when both incontinence and decreased force of stream coexist. The preoperative length of the membranous urethra determined on magnetic resonance imaging (MRI) has been shown to be significantly related to time to postoperative continence. When urethral length was greater than 12 mm, 89% of the patients were continent at 1 year, versus 77% with 12 mm or less than this length (Coakley et al, 2002). Urodynamic studies revealed that a reduced functional urethral length was a predictive parameter of incontinence (Hammerer and Huland, 1997; Van Kampen et al, 1998; Wei et al, 2000). Different components of the urethra may also be involved. The urethral intrinsic component responsible for passive continence, as well as the extrinsic component responsible for active continence, may be involved as has been demonstrated in a study using urodynamic evaluation and alpha blockade (Pfister et al, 2002). This may explain passive incontinence despite a high voluntary urethral pressure or that measured during an active squeeze by the patient. Postoperative disruption of the innervation of the posterior urethra may also be involved and can affect both motor and sensory function (John et al, 2000; Bader et al, 2001). UI occurring after radical prostatectomy (RP) is a significant problem. Although its rate has lessened in recent years primarily due to a better understanding of pathophysiology and improvements in surgical technique, its prevalence has increased due to the dramatic increase of laparoscopic and robotic-assisted laparoscopic radical prostatectomy as standard treatments for men with prostate cancer in developed countries (Hu et al, 2003, 2004) within the past decade. Based on the body of available data on postoperative continence, it would appear that incontinence rates are similar between open and laparoscopic/robotic approaches. Several studies have compared the techniques either prospectively in nonrandomized fashion (Anastasiadis et al, 2003; Jacobsen et al, 2007) retrospectively (Ahlering et al, 2004) or via limited meta-analysis (Rassweiler et al, 2003; Salomon et al, 2004), and similar continence rates were found. Further prospective comparative studies with open surgery are necessary. Perineal prostatectomy is done by only a limited number of urologists but is still advocated for obese patients. The continence rate was reported as similar to the retropubic route (Weldon et al, 1997; Gray et al, 1999; Harris, 2003). Data from large multicenter studies and prostate cancer databases suggest that following RP, 1% to 40% of patients complain of persistent urinary incontinence. The incidence of postprostatectomy incontinence (PPI) depends on the definition of UI and the length of follow-up (Olsson et al, 2001; Krupski et al, 2003; Rodriguez et al, 2006). Recent reports of large cohorts use definitions that include “total control/perfect continence,” “occasional leakage but no pad,” and “less than one pad.” Because one third to one half of men who do not wear pads will have occasional leakage of urine, it is important to distinguish among those men who leak enough to require pad use and those who do not. It has been demonstrated that health-related quality of life is strongly correlated with the level of incontinence, and wearing a pad more significantly affects the quality of life than wearing no pad (Litwin et al, 2000). In addition, not all men who leak will elect to have further treatment. Most large cohort studies indicate that between 6% and 9% of patients undergo subsequent surgical treatment for PPI following prostate cancer surgery (Stanford et al, 2000; Begg et al, 2002; Steineck et al, 2002; Penson et al, 2005). Reported risk factors for incontinence following radical prostatectomy include the following: Preoperative sphincteric insufficiency (demonstrated by either the preexisting clinical sign of SUI or the urodynamic finding of lower maximal urethral closure pressure) predicts postoperative SUI (Wei et al, 2000; Majoros et al, 2006). Preoperative bladder dysfunction can also contribute to postoperative incontinence. Preexisting abnormalities of detrusor function may predispose to leakage following surgery, especially neurogenic detrusor overactivity due to Parkinson disease, dementia, or spinal cord injury (Khan et al, 1991). Advancing age as a risk factor is supported by several studies (Leandri et al 1992; Zincke at al, 1994; Eastham et al, 1996; Catalona et al, 1999; Horie et al, 1999; Moore et al, 2007). Others have found advancing age and the number of comorbidities to have a negative impact on the recovering time for continence during the first year after radical prostatectomy (Young et al, 2003). Rogers and colleagues demonstrated that age affected postoperative continence status following laparoscopic RRP. In those younger than 50 years old, 100% achieved 0 to 1 pad per day continence at 1 year, which decreased to 91% and 81% for those 50 to 59 years old and older than 60 years, respectively (P < 0.01) (Rogers et al, 2006). Strasser and colleagues (1998) hypothesized that age-related sphincteric changes may be responsible for the age-related increase in postoperative SUI and successfully demonstrated a progressive reduction in sphincter striated muscle cells with age. Most large series have found no correlation between the stage of disease and incontinence rates (Eastham et al, 1996; Jonler et al, 1996; Lowe, 1996; Catalona et al, 1999; Pierorazio et al, 2007). However, in certain cases, the stage of disease may affect the surgical technique (i.e., nerve sparing) and incontinence rates may be higher, but this appears to be a reflection on surgical technique and not disease stage (Zincke et al, 1994). Bladder neck preservation has been reported to improve continence at 3 months (Lowe, 1996), but no difference was evident at 6 and 12 months (Poon et al, 2000; Srougi et al, 2001).
Definition and Classification of Urinary Incontinence
Conditions
Definition and Classification of Pelvic Organ Prolapse
Relationship Between Incontinence and Prolapse
Pathophysiology of Incontinence And Prolapse
Continence
Neurogenic Detrusor Overactivity
Idiopathic Detrusor Overactivity
Interpretation of Vesicourethral Dysfunction
Classification of Neuropathic Bladder Dysfunction
Idiopathic Detrusor Overactivity
Bladder Compliance during Filling Cystometry
Neurogenic
Non-Neurogenic (Increased Collagen)
Sphincteric Mechanisms
Male Sphincteric Mechanisms
Female Sphincteric Mechanisms
MALE
FEMALE
Proximal bladder neck mechanism
Powerful
Weak
Distal urethral mechanism
Powerful
Extending along the majority of the length of the female urethra and prone to the effect of exogenous influences such as pelvic floor weakness and damage or denervation consequent on childbirth
Prostate
Further increases bladder outlet resistance
Not present
Urethra
Long
Short (≈3.5 cm)
Conditions Causing Sphincter Abnormalities
Sphincter Abnormalities in Women
Sphincter Abnormalities in Men
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Urinary Incontinence and Pelvic Prolapse: Epidemiology and Pathophysiology
• Increased daytime frequency is the complaint by the patient who considers that he or she voids too often by day (equivalent to the term pollakisuria used in many countries).
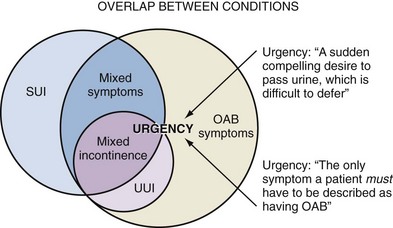
• Urgency is the complaint of a sudden, compelling desire to pass urine, which is difficult to defer.
• Urgency UI is the:
symptomatic complaint of involuntary leakage accompanied by or immediately preceded by urgency as contrasted to urge, which is a normal sensation (Abrams et al, 2009). The current term urge has therefore been replaced by urgency UI.
• Mixed UI is the:
symptomatic complaint of involuntary leakage associated with urgency and also with exertion, effort, sneezing, or coughing.
• Mixed urinary symptoms is a term applied to the presentation of a patient with a combination of OAB dry (without urgency incontinence) and stress incontinence (Fig. 63–2).
• Other types of UI may be situational (e.g., the report of incontinence during sexual intercourse, giggle incontinence). Overflow incontinence is not a symptom or condition but rather a term used to describe leakage of urine associated with urinary retention.
Postmicturition dribble is the complaint of an involuntary loss of urine. Both are voiding symptoms.
Bladder sensation is an important factor to take into account when interpreting storage disorders affecting the lower urinary tract and can be categorized during history taking into five broad categories:
1. Normal: The individual is aware of bladder filling and increasing sensation up to a strong desire to void.
• Pelvic organ prolapse is defined as the descent of one or more of the following: anterior vaginal wall, posterior vaginal wall, and apex of the vagina (cervix/uterus) or vault (cuff) after hysterectomy. Absence of prolapse is defined as stage 0 support; prolapse can be staged from stage I to stage IV.
• Anterior vaginal wall prolapse is defined as descent of the anterior vagina so that the urethrovesical junction (a point 3 cm proximal to the external urinary meatus) or any anterior point proximal to this is less than 3 cm above the plane of the hymen.
• Patients with severe prolapse may develop voiding symptoms as a result of urethral kinking leading to obstruction.
1. An intact normally functioning innervated lower urinary tract (bladder, urethra, sphincters, and pelvic floor)
3. Tonic inhibitory systems in the brain that suppress parasympathetic excitatory outflow to the bladder (Morrison et al, 2005)
• Phasic detrusor overactivity is defined by a characteristic wave form and may or may not lead to urinary incontinence. Phasic detrusor contractions are not always accompanied by any sensation or may be interpreted as a first sensation of bladder filling or as a normal desire to void.
• Neurogenic detrusor overactivity, when there is a relevant neurologic condition. This term replaces the term detrusor hyperreflexia.
• Identify patients who are at risk of deteriorating renal function as a result of the abnormally high bladder pressures
• Supraspinal lesions include all cerebral diseases such as cerebral hemorrhage and thrombosis, dementia, tumors, arteriosclerosis, and Parkinson disease.
• Compliance is calculated by dividing the volume change (ΔV) by the change in detrusor pressure (ΔPdet) during that change in bladder volume (C = V/ΔPdet). It is expressed in mL/cm H2O.
• A variety of means of calculating bladder compliance has been described. The ICS recommends that two standard points be used for compliance calculations, but the investigator may define additional points. The standards points are as follows:
1. the detrusor pressure at the start of bladder filling and the corresponding bladder volume (usually zero), and
• Allow adequate emptying from the bladder with minimal resistance during micturition (voiding phase)
1. A proximal “bladder neck mechanism” consisting of the bladder neck, prostate, and prostatic urethra to the level of the verumontanum. This receives a dual innervation from the autonomic nervous system via parasympathetic and sympathetic fibers. The main motor control is likely to be provided by the sympathetic component, although there is some evidence for a contribution from the detrusor muscle where the predominant innervation is parasympathetic. This portion of the continence mechanism is removed during prostatectomy, leaving only the distal urethral sphincter mechanism to prevent urinary leakage.
2. A distal urethral mechanism at the apex of the prostate. This extends from the verumontanum to the proximal bulb and is composed of a number of structures that help to maintain continence. The male distal sphincter complex is composed of the prostatomembranous urethra, cylindrical rhabdosphincter (external sphincter muscle) surrounding the prostatomembranous urethra, and extrinsic paraurethral musculature and connective tissue structures of the pelvis. The rhabdosphincter is a concentric muscular structure consisting of longitudinal smooth muscle and slow-twitch (type I) skeletal muscle fibers that can maintain resting tone and preserve continence (Gosling et al, 1981; Turner-Warwick, 1983). Skeletal muscle fibers of the rhabdosphincter have been shown to intermingle with smooth muscle fibers of the proximal urethra, suggesting a dynamic and coordinated interaction (Burnett and Mostwin, 1998). The rhabdosphincter is invested in a fascial framework and supported below by a musculofascial plate that fuses with the midline raphe, which is also a point of origin for the rectourethralis muscle (Burnett and Mostwin, 1998). Superiorly, the fascial investments of the rhabdosphincter fuse with the puboprostatic ligaments (Steiner, 2000). This dorsal and ventral support probably contributes to the competence of the sphincter. The striated fibers of the extrinsic paraurethral muscle (levator ani complex), on the other hand, are of the fast-twitch (type II) variety (Gosling et al, 1981). During sudden increases in abdominal pressure, these fibers can contract rapidly and forcefully to provide continence. Continence has been shown to be maintained after inducing paralysis of the striated sphincter (Lapides et al, 1957; Krahn and Morales, 1965), indicating that this structure is not solely responsible for continence in men with an intact functioning bladder neck.
1. Watertight apposition of the urethral lumen. Four urethral wall factors promote continence: (1) wall tension or external compression, (2) inner wall softness, (3) a filler material beneath the mucosa that helps to deform the mucosal folds into apposition, and (4) a lining of mucus provides the stickiness that enables cooptation of these mucosal folds (Zinner et al, 1980). Histologic cross sections of the urethra show that the urethra is not simply a closed tube; rather, there are numerous mucosal folds, between which are potential spaces for urine leakage. In women, estrogen and a cushion effect of the submucosal vasculature have been suggested as ancillary factors (Raz et al, 1972; Tulloch, 1974).
1. Previous urethral or periurethral surgery (e.g., anti-incontinence surgery, urethral diverticulectomy) may result in postoperative ISD from periurethral fibrosis, scarring, or denervation (Haab et al, 1996). Prevalence of ISD after two or more failed anti-incontinence operations was found to be as high as 75% (McGuire et al, 1980).
2. Neurologic insult may cause ISD. Sacral neurologic lesions have a variable effect on micturition, depending on the extent to which the neurologic injury affects the parasympathetic, sympathetic, and somatic systems (Gerstenberg et al, 1980; Blaivas and Barbalias, 1983; Wheeler et al, 1986; McGuire et al, 1988). In complete parasympathetic lesions, the bladder is areflexic and the patient is in urinary retention. When, in addition to a parasympathetic lesion, there is a sympathetic lesion, the proximal urethra loses its sphincteric function. Clinically, this results in incomplete bladder emptying, caused by the acontractile detrusor, and sphincteric incontinence, caused by the nonfunctioning proximal urethra (Gerstenberg et al, 1980; Blaivas and Barbalias, 1983). Somatic neurologic lesions affect pudendal afferent and efferent nerves (Blaivas, 1982). In addition to loss of perineal and perianal sensation, these lesions abolish the bulbocavernosus reflex and impair the ability to contract the urethral and anal sphincters voluntarily. Sacral neurologic lesions are caused by herniated disks, diabetic neuropathy, multiple sclerosis, and spinal cord tumors. They are also commonly encountered after extensive pelvic surgery such as abdominoperineal resection of the rectum and radical hysterectomy (Gerstenberg et al, 1980; Blaivas and Barbalias, 1983; Chang and Fan, 1983; McGuire et al, 1988).





