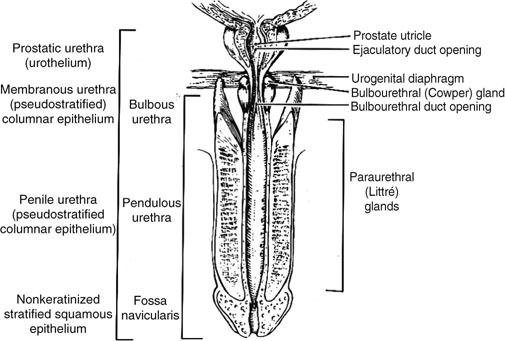
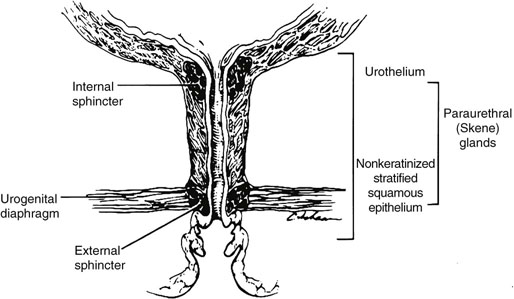
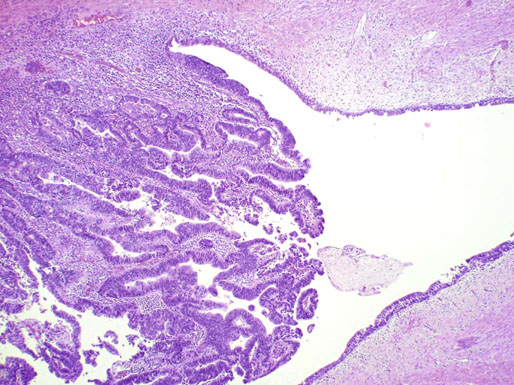
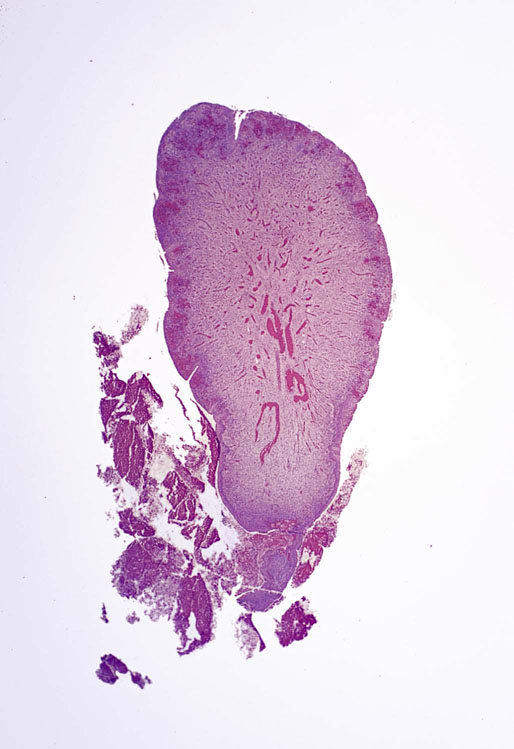
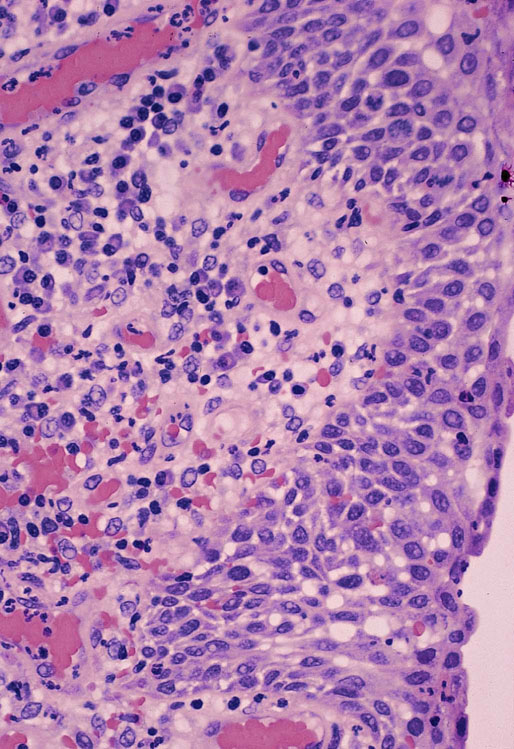
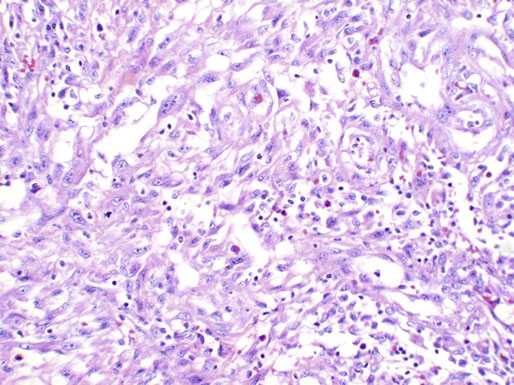
Chapter 11 The urethra conveys urine from the urinary bladder to the exterior through the external urethral meatus. In males, it also serves as a conduit for semen. The epithelium of the urethra is derived from the urogenital sinus, which is formed when the endodermal cloaca divides into the rectum dorsally and the urogenital sinus ventrally, separated by the urorectal septum.1 In females, the epithelium of the urethra is derived from endoderm of the urogenital sinus, whereas the surrounding connective tissue and smooth muscle arise from splanchnic mesenchyme. In males, the epithelium also is derived from the urogenital sinus, except in the fossa navicularis, where it is derived from ectodermal cells migrating from the glans penis. As in females, the connective tissue and smooth muscle surrounding the male urethra are derived from splanchnic mesenchyme. In men, the urethra is 15 to 20 cm long and is divided into three anatomic segments (Fig. 11-1). The prostatic urethra is approximately 3 to 4 cm long and begins at the internal orifice at the bladder neck and extends through the prostate to the prostatic apex.2 Most prostatic ducts open along the posterior and lateral walls of the prostatic urethra adjacent to the urethral crest, the longitudinal ridge along the dorsal wall of the prostatic urethra. In the central part of the urethral crest is an eminence called the verumontanum or colliculus seminalis. The verumontanum contains a slitlike opening that leads to an epithelial-lined sac called the prostatic utricle, a müllerian vestige. The ejaculatory ducts empty into the urethra on either side of the prostatic utricle. The membranous urethra is the shortest segment, only 1 cm long. It extends from the prostatic apex to the bulb of the penis and traverses the musculature of the urethral sphincter and inferior fascia of the urogenital diaphragm. Cowper glands, which are small, paired bulbomembranous urethral glands, are located on the left and right sides of the membranous urethra and secrete into it.2–6 The penile urethra is the longest segment (10 to 15 cm) and extends from the lower surface of the urogenital diaphragm to urethral meatus in the glands penis. The orifices of the bulbomembranous urethral glands are located on the lateral surfaces of the proximal (bulbous) portion of the penile urethra. The penile urethra is surrounded by the corpus spongiosum along its length. Scattered mucus-secreting periurethral glands (Littré glands) are present at the periphery of the penile urethra except anteriorly. The female urethra is approximately 4 cm long (Fig. 11-2). At its periphery are paraurethral glands (Skene glands), which empty into the urethra through two ducts near the external urethral orifice. The type of epithelium lining the urethra varies along its length.2–4 In general, urothelium lines the prostatic urethra; pseudostratified columnar epithelium lines the membranous segment and most of the penile urethra, and nonkeratinized stratified squamous epithelium lines the fossa navicularis and external urethral orifice. In females, the proximal one third of the urethra is lined by urothelium and the distal two thirds by nonkeratinized stratified squamous epithelium. However, most urethral tissues submitted for surgical pathologic examination are diseased or altered by instrumentation, and both circumstances may cause metaplastic changes. The lymphatic drainage of the male urethra arises from a rich mucosal network that extends the entire length of the urethra.5 This network is continuous proximally with that of the prostate and urinary bladder and distally with that of the penis. The lymphatics of the prostatic and bulbomembranous segments drain to the obturator and medial external iliac lymph nodes, whereas those of the distal penile urethra drain to the superficial inguinal nodes. In females, the proximal urethra drains to the external iliac, hypogastric, and obturator lymph nodes. The distal urethral lymphatics communicate freely with vulvar lymphatics and drain to the superficial inguinal nodes. Several congenital anomalies may affect the urethra, but they are rarely encountered by surgical pathologists. Urethral valves are mucosal folds that project into the urethral lumen and may cause obstruction, hematuria, or inflammatory symptoms, although they are usually asymptomatic.7,8 Urethral valves are usually covered by normal urothelium but may be inflamed. The submucosa may also be inflamed and edematous. The so-called posterior urethral valves, usually seen in men, are associated with bladder neck hypertrophy.7 One study reported prenatal diagnosis in monochorionic twins.9 Urethral diverticula are uncommon and are often overlooked or misinterpreted. Most urethral diverticula occur in women.10–13 They may be asymptomatic but can manifest with irritative symptoms or dribbling, sometimes with localized pain.14 On physical examination, diverticula manifest a paraurethral mass that can sometimes be palpated through the vagina. It is thought that urethral diverticula may be either acquired or congenital, but no clear morphologic criteria exist to make this distinction. Most urethral diverticula in adults are acquired as sequelae of infection, trauma, calculi, obstruction, dilation, or inflammation of a paraurethral gland.12,13,15,16 Diverticula are usually lined by urothelium, although they often undergo squamous or glandular metaplasia. Nephrogenic adenoma may also arise in diverticula.17,18 The submucosa often is edematous and inflamed. Most patients with clinically apparent urethral diverticula have a major complication such as infection, stricture,19 lithiasis with subsequent obstruction, or carcinoma (Fig. 11-3).12,20 The percentage of urethral diverticula that develop cancer is unclear, with reported incidences ranging from 2% to 15% of symptomatic diverticula.21,22 Carcinoma that develops in this setting is usually squamous cell carcinoma or adenocarcinoma, but it may also be urothelial.23,24 Adenocarcinoma may be of the conventional type or of the clear cell type (see the section on clear cell adenocarcinoma later in this chapter).25,26 Fig. 11-3 Adenocarcinoma arising in a urethral diverticulum. Note transition from normal urothelium to adenocarcinoma in situ. The main differential diagnostic consideration for diverticulum is urethral cyst.27,28 An unusual case of endometriosis in a woman manifested as diverticulum.29 Duplication of the urethra is rare and usually comes to the attention of the surgical pathologist at autopsy.30–32 The first description of a case of duplication of the urethra is attributed to Aristotle. Duplication of the urethra may be complete, extending from the bladder to the dorsum of the penis,31 or partial, extending from the dorsal surface or, less commonly, the ventral surface of the penis and ending blindly.33 Only 15% of cases of duplicated urethra, whether complete or partial, connect with the functional urethra. Most cases are asymptomatic, but the most common complication is infection.34,35 Patients may have urinary obstruction caused by compression of the functional urethra by a mass of desquamated material in the blind accessory urethra. In other cases, patients may complain of incontinence or double urinary stream. Also known as fibroepithelial polyp, congenital urethral polyp, a rare lesion, occurs almost exclusively in males.36–44 Patients usually come to clinical attention between the ages of 3 and 9 years, but rarely they may present during infancy or adulthood.7 For this reason, it has been suggested that congenital urethral polyp occurs secondary to a poorly understood congenital defect in the urethral wall. Congenital urethral polyp usually arises in the prostatic urethra adjacent to the verumontanum (posterior urethral polyp). Signs and symptoms include hematuria, difficulty voiding, urinary retention, and infection. Symptoms are similar to those of other obstructing urethral lesions, including urethral valve, stricture, and lithiasis. Morphologically, congenital urethral polyp is covered by urothelium that may be inflamed, ulcerated, or exhibit squamous metaplasia. This differs from the more common prostatic urethral polyp occurring in adults that is covered by prostatic epithelium (see the later section on ectopic prostatic tissue and prostatic urethral polyp). Anterior urethral polyp is extremely rare and arises in the membranous or penile urethra.42 It produces the same symptoms and has the same morphology as posterior polyp. The subepithelial stroma consists of loose fibrous tissue that may be highly vascular and may contain a few fascicles of smooth muscle. If it has a long stalk, it may “telescope” into the bladder and produce bladder outlet obstruction. Polyps in prepubertal girls and women probably arise from prolapsing urothelium that has evolved into a polyp.45 Urethritis is defined morphologically as an inflammatory response within the urethra. Men are often asymptomatic, and the diagnosis is made by the presence of a urethral discharge and the finding of neutrophils in the urethral smear. Women are often symptomatic; the symptoms are similar to those of cystitis, including dysuria, urinary urgency, and urinary frequency.46–48 A urethral smear will also aid in the diagnosis in women. Urethritis may be caused by sexually transmissible agents such as Neisseria gonorrhoeae, Chlamydia trachomatis, Gardnerella vaginalis, Ureaplasma urealyticum, Mycoplasma hominis, Trichomonas vaginalis, and Candida species. In women, urethritis secondary to Neisseria, Trichomonas, or Candida rarely occurs without concomitant cervical infection.47 Reiter syndrome is characterized by the triad of urethritis, conjunctivitis, and arthritis.49 The etiology is uncertain, but it is usually preceded by an enteric or venereal infection. The syndrome occurs predominantly in men between the ages of 18 and 40 years, but women occasionally are affected. Urethritis is the most common initial symptom. Other urologic manifestations of Reiter syndrome include prostatitis and hemorrhagic cystitis. In the acute phase, the mucosa appears congested and may contain shallow ulcers. Symptoms commonly subside within 2 to 4 weeks, but they recur at irregular intervals in 50% to 75% of cases. Not all involved organ systems may be symptomatic at the same time, so this syndrome should always be included in the differential diagnosis of urethritis in young adults. Urethral caruncle is a pedunculated or sessile polypoid lesion in women that is located in the distal urethra near the meatus. Grossly it has a fleshy, pink-red appearance and bleeds readily (Fig. 11-4). Patients may be asymptomatic, although commonly they experience dysuria, urinary frequency, or obstructive symptoms.50–53 Three histologic subgroups are described: papillomatous, angiomatous, and granulomatous. This distinction is based on the most prominent component (surface epithelial, vascular, and inflammatory, respectively), but it has no apparent clinical relevance. The surface epithelium may be urothelial or squamous and is invariably inflamed (Fig. 11-5); caruncle covered by metaplastic columnar epithelium has been reported. The epithelium may be hyperplastic and constitutes the bulk of the lesion. The underlying stroma is richly vascular and inflamed, occasionally containing glandular elements thought to be derived from Skene glands. Fig. 11-4 Whole-mount appearance of caruncle. This reactive erythematous polypoid mass may be confused with a true neoplasm at the time of urethroscopy. Fig. 11-5 Caruncle. Inflamed mucosa and lamina propria with extravasated red blood cells and prominent vascularity. Rarely, the stroma of urethral caruncle may contain atypical mesenchymal cells, mimicking sarcoma (pseudosarcomatous fibromyxoid tumor) (Fig. 11-6).54 The mixed inflammatory infiltrate and rich vascularity, in combination with the clinical setting, should establish the correct diagnosis. Pseudosarcomatous fibromyxoid tumor may appear spontaneously or follow a pelvic surgical procedure by weeks or months (postoperative spindle cell nodule) and may manifest not only as a polypoid lesion but also as a paraurethral mass.55 As in other pseudosarcomatous lesions involving the urothelial tract, the atypical spindle cells are reactive myofibroblasts, which may display cytokeratin, actin, immunoglobulin G4, and anaplastic lymphoma kinase (ALK) immunoreactivity.56,57 Epithelial membrane antigen results are invariably negative. Electron microscopy is very helpful in establishing the myofibroblastic component. Fig. 11-6 Pseudosarcomatous fibromyxoid lesion (postoperative spindle cell nodule). The patient developed a hemorrhagic polypoid mass several months after an endoscopic procedure. Note myofibroblasts with epithelioid nuclei and abundant eosinophilic cytoplasm, scattered inflammatory cells, and prominent vascularity. Polypoid urethritis is the urethral counterpart of polypoid cystitis, although an association with indwelling catheter has not been noted with urethral lesions.58,59 Polypoid urethritis is a nonneoplastic inflammatory lesion that usually resolves spontaneously after removal of the inflammatory stimulus. It is commonly found in the prostatic urethra near the verumontanum, and it appears as single or multiple polypoid or papillary growths. Morphologically, it is characterized by abundant edematous stroma containing distended blood vessels and a chronic inflammatory infiltrate (Fig. 11-7). The overlying urothelium may be ulcerated or exhibit metaplastic and proliferative changes such as squamous metaplasia, Brunn nests, or urethritis cystica.58,59 Fig. 11-7 Polypoid urethritis. This reactive fibroepithelial lesion results from chronic local insult. Similar to Brunn nests and urethritis cystica, nephrogenic adenoma is a reactive, proliferative lesion that may occur anywhere along the urothelial tract as a consequence of local irritation.60–63 It is most common in the urinary bladder, but it occasionally arises in the urethra. Nephrogenic adenoma is thought to arise through metaplasia of the urothelium in response to an inflammatory stimulus or local injury, and some researchers prefer the term nephrogenic metaplasia. Often this lesion is an incidental finding at surgery for other reasons. The most common symptom is hematuria. This lesion appears grossly as flattened, erythematous areas or as discrete papillae. Microscopically, the latter architecture consists of complex papillary structures covered by cuboidal epithelium with basophilic or eosinophilic cytoplasm, which may be vacuolated. The nuclei are round to oval, hyperchromatic, and centrally located, and they may contain small nucleoli. Mitotic figures are uncommon. The same epithelium may form discrete tubules in the underlying stroma. These tubules have distinct lumina that are usually empty but may contain deeply eosinophilic secretions or pale basophilic material (Fig. 11-8). The tubules are thought to arise through a process of invagination from the surface epithelium, much like Brunn nests. Each is surrounded by a distinct basement membrane.60 Infrequently, cuboidal cells are present in the stroma singly or in small groups lacking a visible lumen, or they may have a signet ring cell appearance. The luminal secretions may be periodic acid–Schiff positive, diastase resistant, or mucicarminophilic, but intracytoplasmic mucin is less frequent. In a study of 26 cases of nephrogenic adenoma involving the prostatic urethra, Allan and Epstein found that 77% of cases extended into smooth muscle, a finding that is not surprising given the anatomy of this site.64 The lesion often appears infiltrative and may be confused with adenocarcinoma, especially in cases lacking a papillary component and composed primarily of tubules in the stroma. The surrounding stroma may be edematous and inflamed, but there is no desmoplastic reaction to the epithelial cells. Allan and Epstein reported focal immunoreactivity for prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP) in 36% and 55% of cases, respectively. However, strong cytokeratin 7 positivity, combined with attention to the cytomorphologic features, should be sufficient to arrive at the correct diagnosis.64 Fig. 11-8 Nephrogenic adenoma. A, Low-power magnification. B, High-power magnification. Note the small tubules, which may be confused with adenocarcinoma. No convincing evidence indicates that nephrogenic adenoma is a preneoplastic condition, although rare cases coincidentally coexist with or precede the development of carcinoma.62 Nevertheless, it is possible that both have common predisposing conditions and consequently may develop independently. For example, nephrogenic adenoma and adenocarcinoma have been reported in association with urethral diverticulum.18 Like other proliferative lesions of the urothelium, nephrogenic adenoma may recur after resection if the inflammatory stimulus is not removed. Malakoplakia is a rare condition that mainly affects the urothelial tract but has also been described in other sites such as the testes, gastrointestinal tract, and retroperitoneum.65–67 Although it may occur anywhere along the urothelial mucosa, most cases occur in the urinary bladder, and urethral involvement is rare.68–71 Women are affected more often than men in a ratio of 4 : 1. Patients usually present with irritative symptoms or urinary obstruction, and endoscopy may reveal an erythematous, plaquelike lesion or polypoid or nodular mass that is clinically suggestive of neoplasm. Microscopically, malakoplakia is characterized by a mixed inflammatory infiltrate dominated by histiocytes with abundant granular, eosinophilic cytoplasm (von Hansemann cells). The cytoplasm contains Michaelis-Gutmann bodies, laminated calcospherites that are basophilic and targetoid in appearance, measuring 5 to 10 µm in diameter. These stain for iron as well as calcium and may occasionally be found within the stroma. The overlying urothelium may be ulcerated, hyperplastic, or metaplastic. In chronic lesions, the characteristic infiltrate may be replaced by fibrosis and scar. The etiology of malakoplakia is unknown, although current knowledge suggests that it is an unusual response to infection, perhaps the result of a disturbed immune response or abnormal macrophage or lysosomal function in the host.65–67 The urothelial tract can be involved in cases of systemic amyloidosis but rarely is the primary site of disease.72–77 In descending order of frequency, amyloid deposits have been described in the urinary bladder, ureter, renal pelvis, and urethra. The usual clinical presentation is hematuria, although dysuria, partial obstruction, or a deviated urinary stream has also been reported. At cystoscopy, the lesion may appear anywhere along the urethra as an elevated plaque or mass that is commonly confused with neoplasm. The overlying mucosa may be ulcerated or hyperemic. The amyloid deposits appear as eosinophilic, homogenous material within the lamina propria, and they often extend into the underlying muscle and connective tissue. Perivascular amyloid deposits are uncommon in tumoral amyloidosis but common in systemic amyloidosis. Inflammation is usually absent except adjacent to ulcerated mucosa. Special stains, such as Congo red, crystal violet, or van Gieson solution of trinitrophenol and acid fuchsin, are useful in establishing the diagnosis. Localized lesions may be managed by transurethral resection, but cases with diffuse involvement and intractable symptoms may require radical surgery.78 Condyloma acuminatum is a common sexually transmitted infectious squamoproliferative growth caused by human papillomavirus that is not related to squamous papilloma.79 It usually occurs on the mucocutaneous surfaces of the external genitalia, perineum, or anus, but extension into the urethra occurs in up to 20% of cases.80–83 It is often multifocal or diffuse. Macroscopically, condyloma is smooth, pink tan, and often papillary. Flat condylomata may be difficult to visualize cystoscopically. Microscopically, it consists of papillary fronds or flat mucosa containing hyperplastic squamous epithelium that may be hyperkeratotic. The squamous epithelial cells typically have clear perinuclear halos, and the nuclei are eccentrically placed, hyperchromatic, and pleomorphic (koilocytic atypia) (Fig. 11-9). Many cases can be diagnosed by these morphologic features alone, although in subtle cases the diagnosis can be confirmed by immunohistochemistry, viral culture, in situ hybridization, or polymerase chain reaction.84–87 The antibodies currently available to identify human papillomavirus are rather insensitive; in situ hybridization and polymerase chain reaction are more sensitive and specific than morphology, even from paraffin-embedded sections.88 The human papillomavirus serotypes most commonly found in urothelial condylomata are 6, 11, 16, and 18, although high-risk genotypes usually predominate.83 These often coincide with the type in the patient’s sexual partner.83–86,89 Fig. 11-9 Condyloma acuminatum. Cells contain vacuolated cytoplasm and irregular nuclei with perinuclear halos. Condyloma of the urinary tract may cause hematuria and irritative symptoms. Surgical management may include transurethral resection, laser, cryotherapy, or a more radical procedure, depending on the extent of disease. Condylomata may undergo transformation to verrucous or infiltrating squamous cell carcinoma.82,85,86 Urothelium frequently undergoes squamous or glandular metaplasia as a response to chronic inflammatory stimuli such as urinary tract infection, diverticula, calculi, or repeated instrumentation (see Fig. 11-3). This situation is very common and is not preneoplastic per se. Nevertheless, under certain conditions, carcinoma may arise in metaplastic epithelium, as in adenocarcinoma or squamous carcinomas arising in diverticula. Glandular metaplasia is more common in the urinary bladder, but it may occur along the urethra. The morphology of the metaplastic urothelium is usually tall columnar with goblet cells, strikingly similar to enteric epithelium. Prostatic acinar epithelium may line the urothelial tract focally. This is seen mostly in men but occasionally occurs at younger ages.90–95 This process is most common in the prostatic urethra (prostatic urethral polyp) but has also been described at the bladder neck and bulbous and penile urethra.96–99 This ectopic tissue is usually asymptomatic and is discovered at urethroscopy for other causes. Hematuria is the most common symptom. Cystoscopically, the lesions appear as discrete, small, papillary growths that may be solitary or extensive, producing a velvety coating of the mucosa (Fig. 11-10). The papillary fronds contain a thin fibrovascular core and are covered by prostatic acinar epithelium with abundant clear or faintly eosinophilic apical cytoplasm and small, basally located, round or oval nuclei without visible nucleoli (Fig. 11-11). Occasionally, foci of residual urothelium are intermingled with the prostatic epithelium. Immunohistochemical stains for PSA are positive.92,94,96 Fig. 11-11 Prostatic urethral polyp. The urothelium of the prostatic urethra is replaced by papillary fronds lined by benign prostatic acinar cells. The etiology of this phenomenon is controversial. Prostatic urethral polyp probably results from hyperplasia and overgrowth of the overlying urothelium by prostatic acinar epithelium. It is important to examine the underlying prostatic urethral tissue carefully because there may be an associated acinar-type prostatic adenocarcinoma. Also, the cytologic features of epithelial cells must be evaluated because prostatic adenocarcinoma may extend to the mucosal surface and take on a papillary growth pattern. Rarely, low-grade papillary adenocarcinoma of the bladder or urachus may seed the prostatic urethra and mimic prostatic urethral polyp (Fig. 11-12). The origin of ectopic prostatic tissue in the penile urethra is less clear and may represent implantation, metaplasia, or an embryologic abnormality (Fig. 11-13). These lesions are benign and, if symptomatic, should be managed conservatively by urethroscopic resection or electrocauterization. Urologists commonly see these lesions during endoscopic evaluation for other causes and seldom perform biopsies on them unless the lesions are believed to be the source of the patient’s symptoms. Fig. 11-12 Urethral implant from urachal adenocarcinoma. After partial cystectomy the patient developed urethral mucosal implants, which were treated by transurethral resection. Given the low-grade appearance of this lesion, it was confused with a prostatic urethral polyp until the pathologist compared it with the original lesion and performed immunohistochemical stains for prostate-specific antigen, results of which were negative. Papilloma, like other papillary urothelial tumors, rarely arises de novo within the urethra.100 The definition of papilloma has evolved over the years.101–104 This benign tumor is characterized by discrete, exophytic, papillary projections with thin fibrovascular cores covered by urothelium indistinguishable from normal urothelium. The urothelial cells maintain their polarity perpendicular to the basement membrane and exhibit abundant eosinophilic cytoplasm, which commonly contains perinuclear vacuoles. Nuclei are elongate or round, depending on the plane of sectioning; they may be slightly enlarged compared with normal urothelium, but they show little or no pleomorphism. The chromatin pattern is homogeneous, and nucleoli are absent or small and sparse. Mitotic figures are usually absent, although a few normal mitotic figures may be observed in the basal layer. The thickness of the epithelium (the number of cell layers) varies by the plane of sectioning. Umbrella cells may be prominent and hyperchromatic.101,102,104 Inverted papilloma rarely occurs along the urethra, but when it does it shares all the morphologic features of the more common vesicular inverted papilloma.105,106 Patients usually have hematuria, and on urethroscopy the lesion appears as a polypoid or nodular growth with a smooth, glistening surface. It ranges up to 2.5 cm in diameter,107,108
Urethra
Embryologic development and normal anatomy
Congenital anomalies
Urethral valves
Urethral diverticula
Duplication of the urethra
Congenital urethral polyp
Nonneoplastic diseases
Urethritis
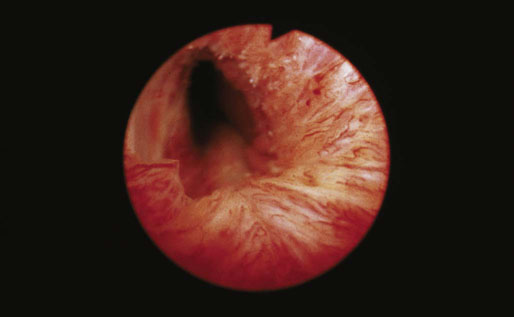
Caruncle
Polypoid urethritis
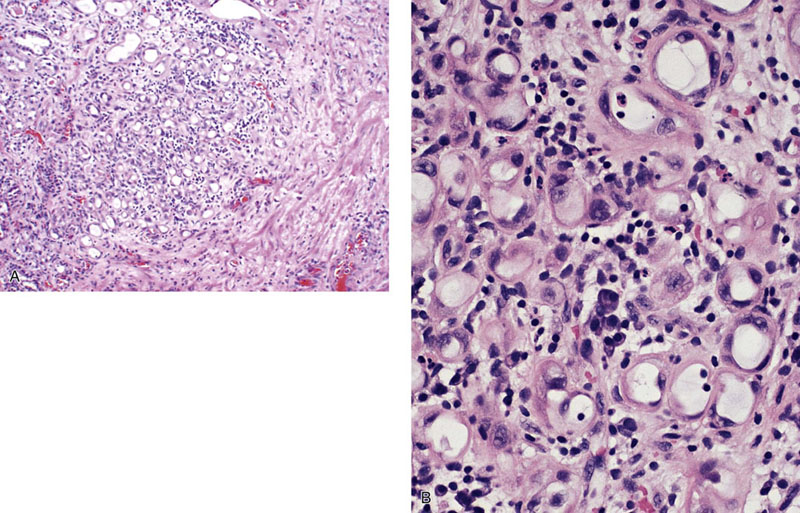
Nephrogenic adenoma (metaplasia)
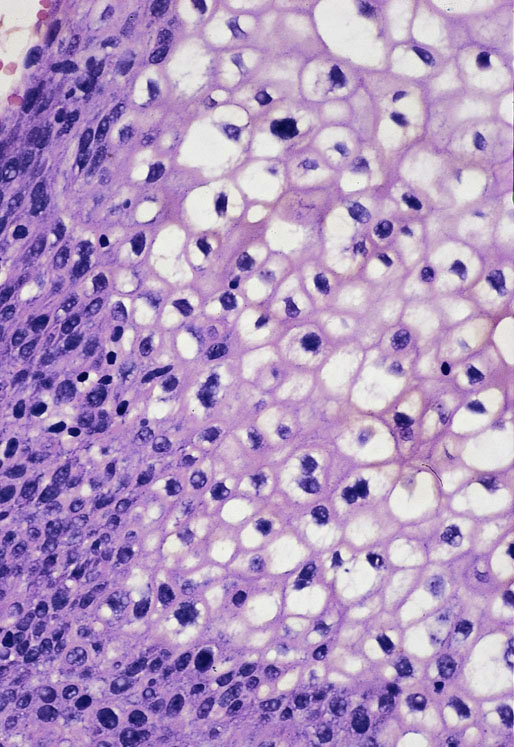
Malakoplakia
Amyloidosis
Condyloma acuminatum
Metaplasia of the urothelium
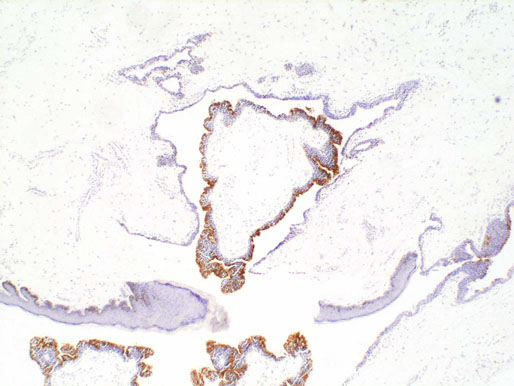
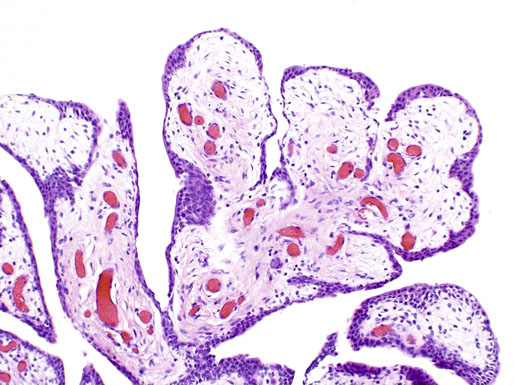
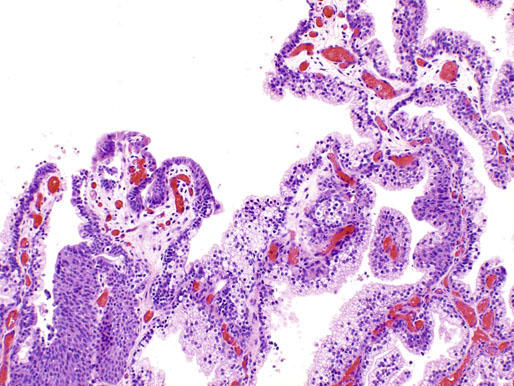
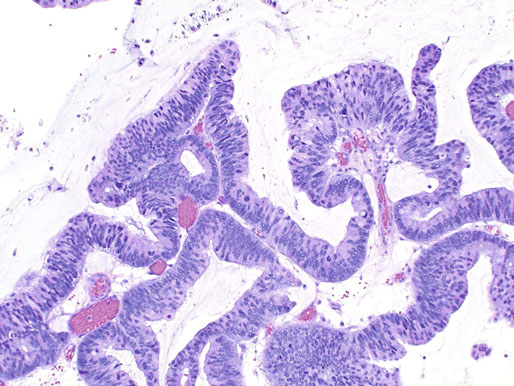
Ectopic prostatic tissue and prostatic urethral polyp
Neoplastic diseases
Benign neoplasms
Papilloma
Inverted Papilloma
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Urethra