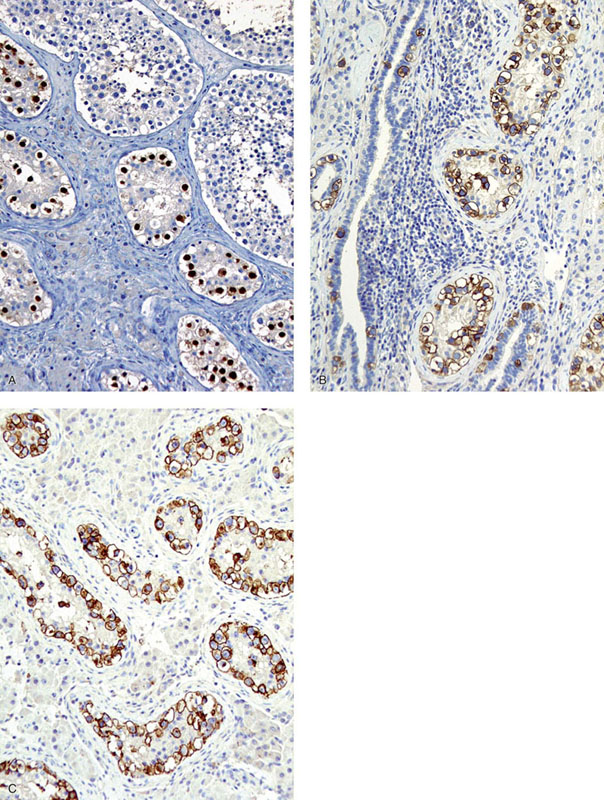
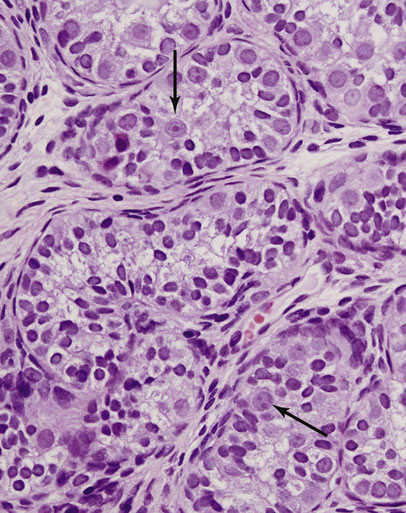
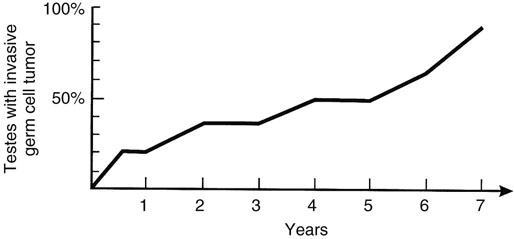
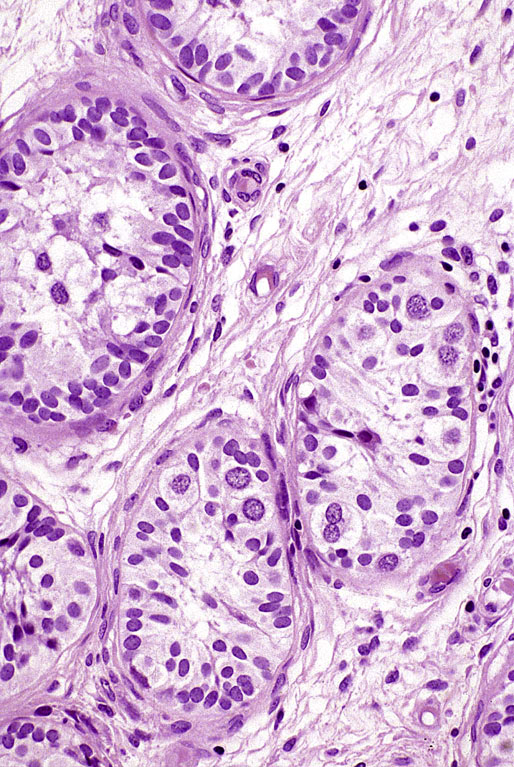
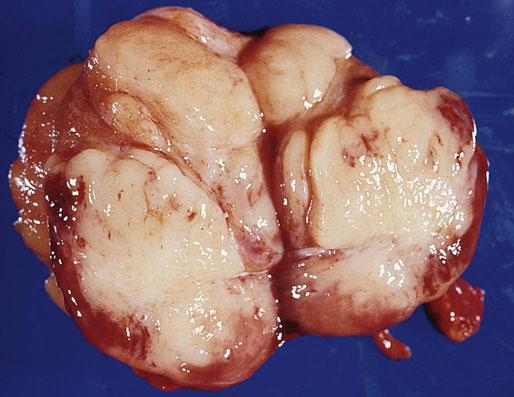
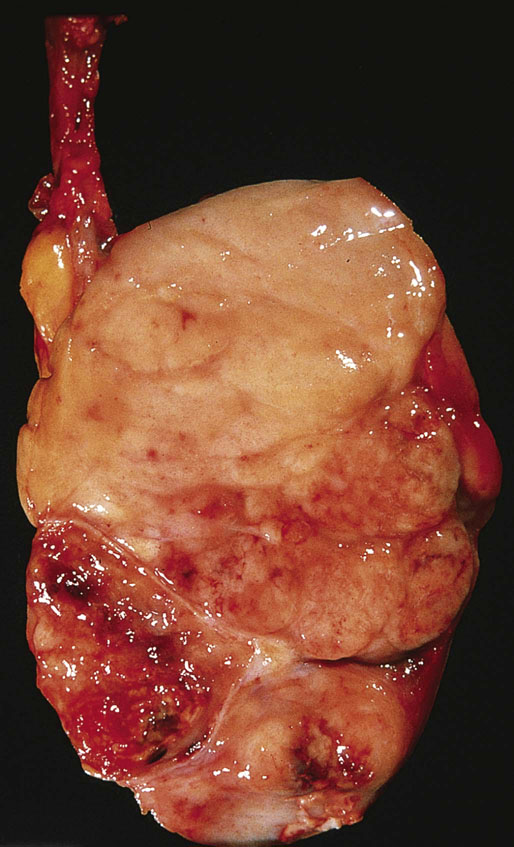
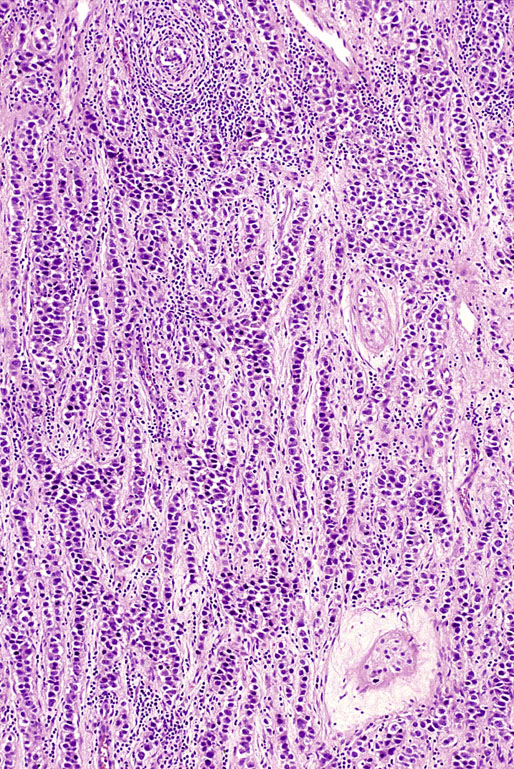
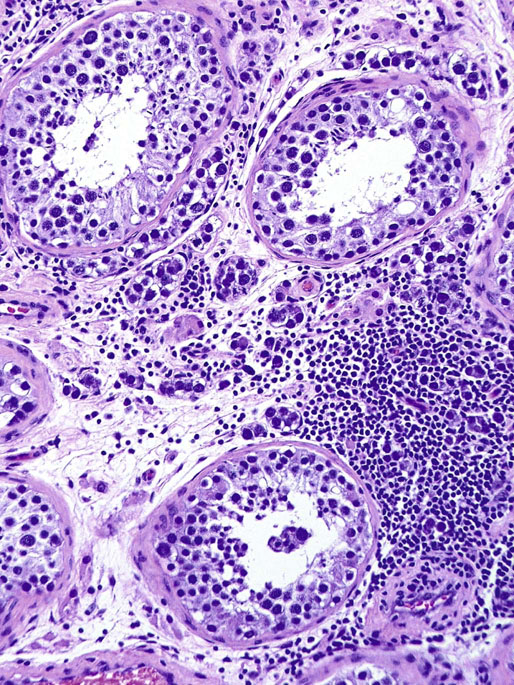
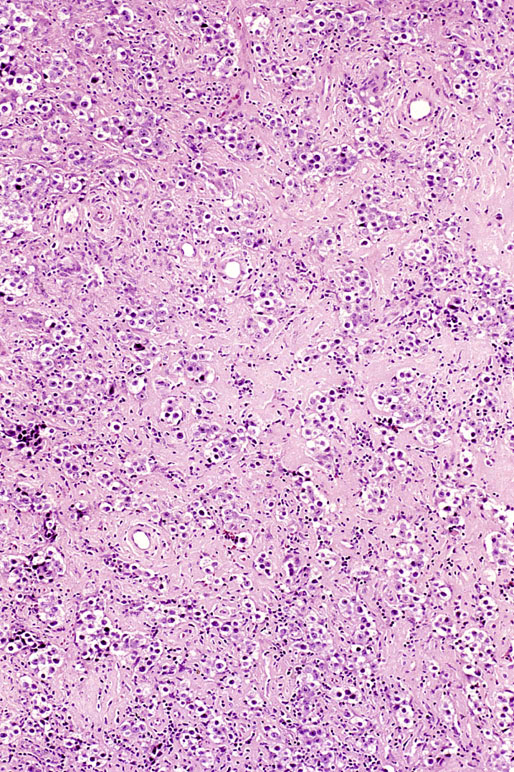
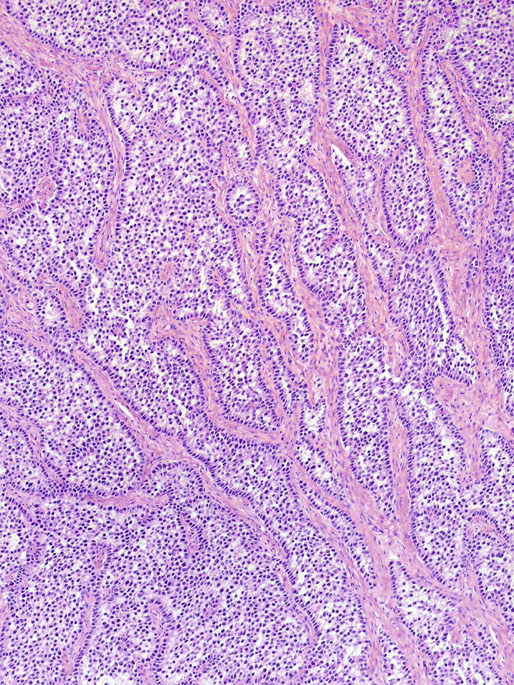
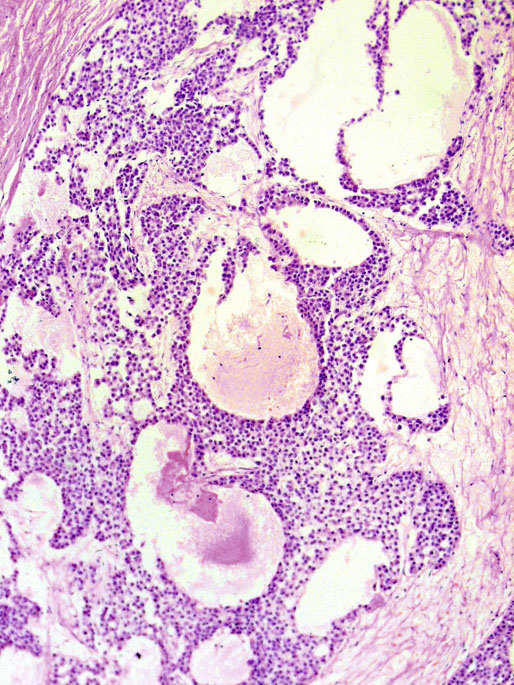
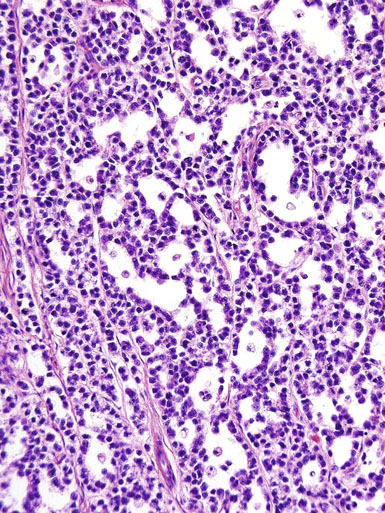
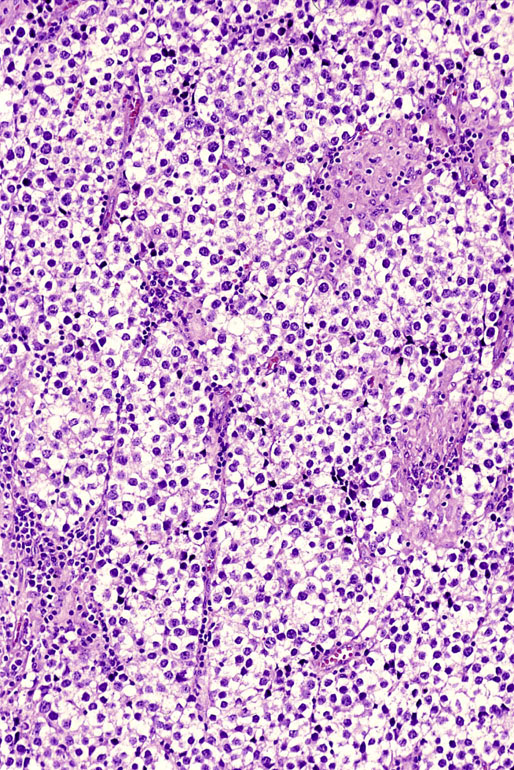
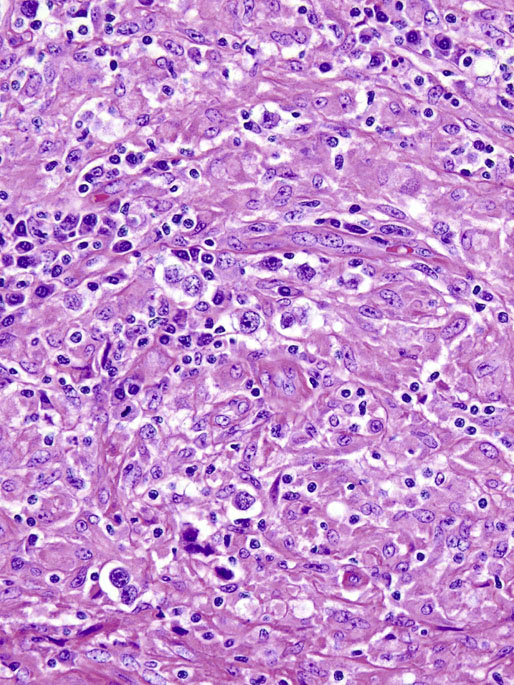
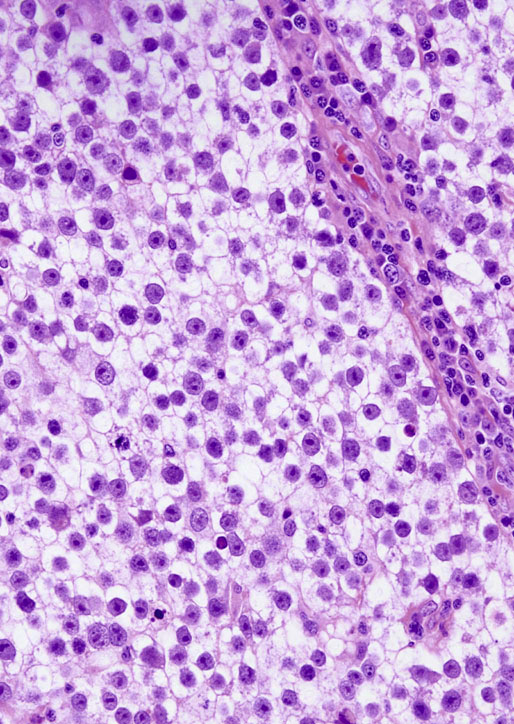
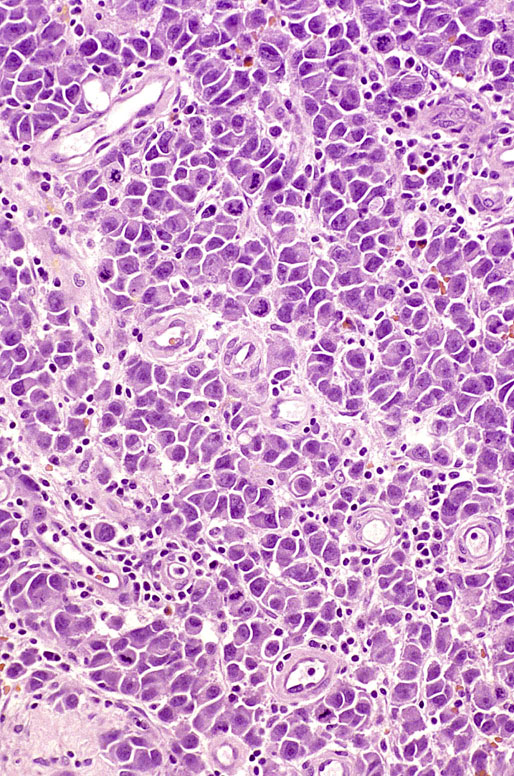
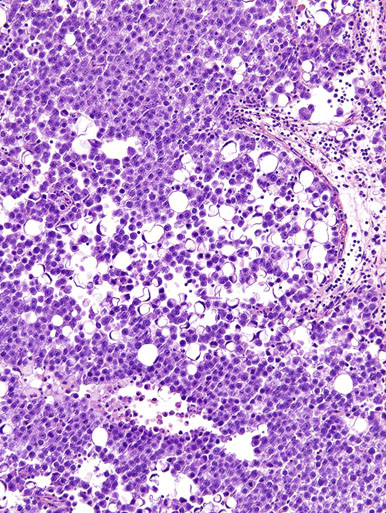
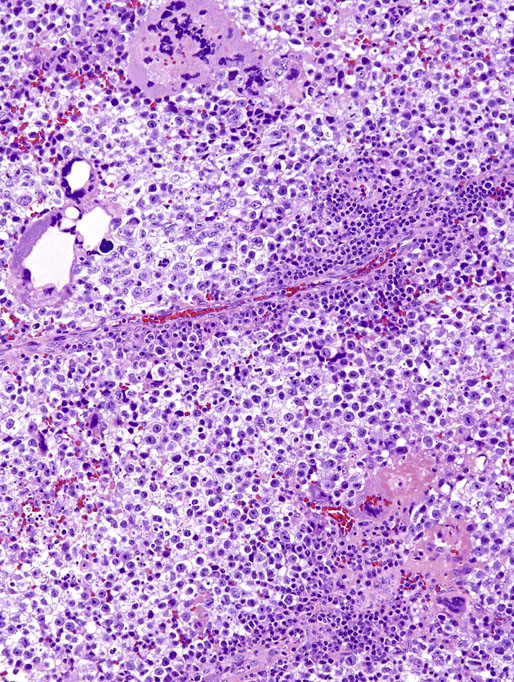
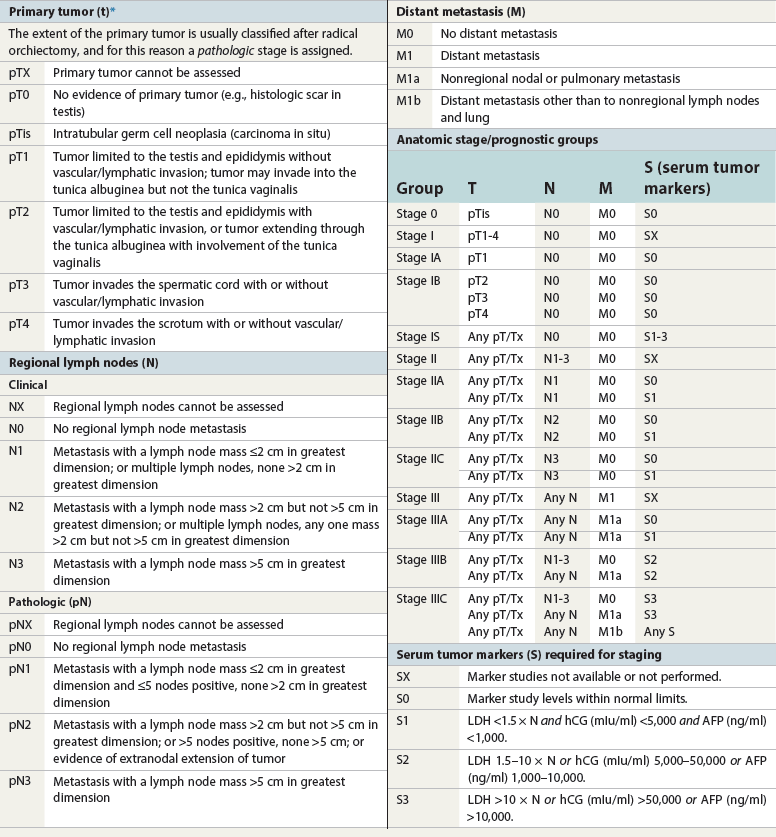
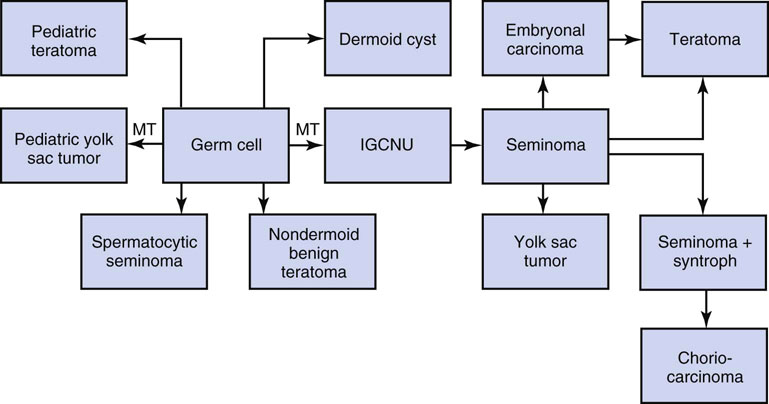
Chapter 13 Thomas M. Ulbright and Robert E. Emerson Chapter outline Intratubular germ cell neoplasia Spermatocytic seminoma with sarcoma Teratoma with a secondary malignant component Choriocarcinoma and other trophoblastic neoplasms Polyembryoma and diffuse embryoma Regression of germ cell tumor (“burnt-out” germ cell tumor) Mixed germ cell and sex cord–stromal tumors Ovarian-type epithelial tumors Neoplasms and tumor-like lesions of the rete testis Neoplasms of lymphoid and hematopoietic cells Although weighing only approximately 19 g,1 the testis is responsible for a complex array of neoplasms. The rapidly proliferating spermatogenic cells give rise to the majority of testicular tumors, 95% of which are of germ cell derivation. Most are malignant and usually occur in young men, but they can be cured by current therapies; therefore accurate diagnosis is essential. The supporting cells and interstitial cells of the testis are responsible for the uncommon sex cord–stromal tumors that represent a disproportionate number of diagnostic problems. Some of these are associated with clinical syndromes that may be detected from the testicular pathology.2–7 Some tumors of soft tissue origin may be identified in the paratestis, and secondary tumors are relatively frequent in both the testis and paratestis. The spectrum of lesions and the capacity of many tumors to mimic others make testicular neoplasia a continuing challenge to surgical pathologists, and this topic has been the subject of several recent reviews.8,9 The currently recommended staging system for testicular cancer is that of the American Joint Committee on Cancer, published in 2010.10 Its subdivisions are shown in Table 13-1. Serum marker studies play a key role in the evaluation of patients with testicular germ cell tumors, so the postorchiectomy values of serum α-fetoprotein (AFP), human chorionic gonadotropin (hCG), and lactate dehydrogenase (LDH) were incorporated into the determination of the stage groupings. Table 13-1 American Joint Committee on Cancer staging system for testicular cancer AFP, α-fetoprotein; hCG, human chorionic gonadotropin; LDH, lactase dehydrogenase; N, the upper limit of normal for the LDH assay. *Note: Except for pTis and pT4, extent of primary tumor is classified by radical orchiectomy. TX may be used for other categories in the absence of radical orchiectomy. From Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer staging manual. New York: Springer, 2010. Testicular neoplasms usually metastasize first to retroperitoneal lymph nodes. Selective lymph node involvement tends to occur with early-stage tumors, depending on whether the right or left testis is involved. For right-sided tumors, the interaortocaval nodes at approximately the level of the second lumbar vertebra are usually first involved, although right paracaval and precaval involvement also may occur.11,12 In early-stage involvement from right-sided tumors, both suprahilar nodal involvement and involvement of the left paraaortic nodes below the inferior mesenteric artery are absent (Fig. 13-1).12 For left-sided tumors, the left paraaortic nodes, in an area bounded by the left ureter, left renal vein, aorta, and origin of the inferior mesenteric artery, are first involved.11 Suprahilar nodal metastases may be seen in early-stage disease from left-sided testicular tumors, in contrast to right-sided lesions (Fig. 13-2).12 As metastases become more widespread, right-sided lesions develop suprahilar and contralateral spread and left-sided tumors develop interaortocaval and precaval involvement and a greater frequency of suprahilar involvement. As the volume of retroperitoneal disease increases, retrograde involvement of iliac and inguinal nodes may be seen.12 Inguinal lymph node involvement also may be seen when the primary tumor has extended to the scrotal skin or a trans-scrotal approach was used for the primary resection. The extension of the primary tumor to the epididymis also correlates with the development of external iliac nodal spread. Eventually, supradiaphragmatic spread occurs to the mediastinum and supraclavicular and cervical lymph nodes, tending to involve the left supraclavicular nodes much more commonly than the right.13 Seminoma tends to metastasize in an orderly pattern through lymphatics, whereas choriocarcinoma more frequently spreads by hematogeneous routes. The other germ cell tumors, such as embryonal carcinoma, tend to have a lymphatic pattern of spread, although hematogeneous spread also can be seen. Hematogeneous spread is most commonly reflected by lung, liver, central nervous system, and bone involvement; brain involvement is most common with choriocarcinoma and, perhaps unexpectedly, bone involvement with seminoma.14 Gross examination and proper handling of the orchiectomy specimen are often neglected, and many diagnostic problems at the microscope can be traced to suboptimal processing of the gross specimen. Under the best circumstances, the testis and accompanying tunics and spermatic cord should be received fresh, dissected, and allowed to thoroughly fix before tissue blocks are submitted. What often happens, however, is that the urologist places the radical orchiectomy specimen intact into fixative, and only hours later is the specimen dissected. The testicular tunics do not permit ready penetration of fixative, so this approach results in autolytic changes. It would be preferable for the urologist to make a single, virtually through-and-through incision in the specimen before placing it into fixative if it is not feasible to send it to the laboratory immediately in the fresh state. A radical orchiectomy specimen consists of the testis, tunica vaginalis, and portion of spermatic cord. The specimen should be weighed and measured in three dimensions, and the cord length should be noted. We recommend examination of the spermatic cord next, prior to incision of the testis, to avoid the common contamination by “buttered” tumor of the cord,8,15 with submission of the cord resection margin and a cross section adjacent to the testis. The tunica vaginalis should then be incised, any abnormalities described, the quantity and nature of any intratunical fluid recorded, and the tunica albuginea carefully inspected and palpated for penetration by neoplasm. The testis should then be bisected in the plane of its long axis, through the testicular hilum, by a long, sharp knife. Fresh tissues may be harvested for special studies, such as cytogenetics, flow cytometry, electron microscopy, and molecular studies, although these are not routinely needed for diagnosis. Photographs may be obtained, and then multiple, serial, parallel cuts at 3-mm intervals should be made, leaving the tunica albuginea intact posteriorly to keep the specimen together. The specimen should be placed in a generous volume of the pathologist’s preferred fixative (10% neutral buffered formalin is quite suitable) and allowed to thoroughly fix prior to further processing. After fixation, the neoplasm should be described and measured, with particular attention paid to the relationships to the tunica albuginea and the testicular hilum. Most examples of extratesticular spread occur by extension through the hilum.16 Multiple blocks of neoplasm should be submitted, because many tumors are quite heterogeneous. Blocks of all of the different-appearing areas should be made, including hemorrhagic and necrotic areas. A minimum of one block of neoplasm for every centimeter of maximum tumor dimension is a general rule of thumb. However, it is prudent to submit blocks quite generously if the gross appearance suggests seminoma, because the discovery of nonseminomatous elements usually changes therapy. Hence, small seminomas should be submitted totally and at least 10 blocks of larger tumors, (or one block for every centimeter of maximum tumor dimension, whichever is the larger number) submitted. The nonneoplastic testis also should be sampled, as well as a block to include the testicular hilum. The epididymis should be incised by multiple parallel cuts perpendicular to its long axis, any abnormalities noted, and the appropriate blocks submitted. Approximately 95% of testicular neoplasms are of germ cell origin. The classification of testicular germ cell tumors in Table 13-2 is a modification of that of the World Health Organization (WHO)17 and is the one recommended, although a second classification developed by the British Testicular Tumour Panel (BTTP)18 is sometimes used in Europe. The histogenesis of testicular germ cell tumors has been clarified over the past 4 decades by clinical, morphologic, and immunohistochemical observations. In the past decade, gene expression profiling19 and other molecular techniques have allowed a great increase in understanding of the molecular changes in the germ cell tumors that most commonly are found in young men, the so-called type II germ cell tumors, reviewed elsewhere.20,21 Perhaps foremost in importance is the recognition that all of the adult germ cell tumors, with the exceptions of spermatocytic seminoma, epidermoid and dermoid cysts, and very rare benign teratomas,22 are derived from a common precursor, which Skakkebaek23 originally recognized and described as “carcinoma in situ” of the testis.24 The preferred term for this lesion, given the nonepithelial nature of the constituent cells, is intratubular germ cell neoplasia of the unclassified type (IGCNU).25,26 It consists of an initially basilar proliferation of seminoma-like cells with clear cytoplasm and enlarged, hyperchromatic nuclei having one or two prominent nucleoli (Fig. 13-3). Additionally, apart from its light microscopic appearance, it shares many features with seminoma, including ultrastructure,27,28 immunohistochemical reactions for various antigens (M2A/podoplanin/D2-40,29 TRA-1-60,30 placental-like alkaline phosphatase (PLAP),31–33 glutathione-S-transferase (isoenzyme π),34 the 14-3-3 β protein,35 OCT4,36,37 NANOG,38 stem cell factor,39 and TSPY protein,40 an SOX17+/SOX2− immunophenotype,41,42 DNA content,43 number of nucleolar organizer regions,44 and lectin binding patterns.45 The strong similarities between IGCNU and seminoma imply that seminoma is also a precursor for other germ cell tumors. This interpretation is supported by morphologic, immunohistochemical, and molecular observations. First, pure testicular seminoma may subsequently develop nonseminomatous elements, as evidenced by histologic examination or elevation of serum AFP. Autopsy studies of patients who died of metastatic germ cell neoplasm after orchiectomy with pure testicular seminoma have demonstrated nonseminomatous elements in 30% to 40% of cases.14,46 By light microscopy, seminoma may undergo subtle transition to either embryonal carcinoma or yolk sac tumor.47,48 Also, 10% to 20% of seminomas contain syncytiotrophoblast cells, and some trophoblastic hormone-containing cells in seminoma are not easily distinguished histologically from the surrounding seminoma cells.49–51 Ultrastructural studies of seminoma have demonstrated evidence of epithelial differentiation (seminoma with early carcinomatous features) in some light microscopically typical cases.52 Furthermore, the DNA content of seminoma is consistently higher than in nonseminomatous germ cell tumors,43,53,54 suggesting that nonseminomatous tumors evolve from seminoma as a consequence of gene loss, perhaps because of loss of cancer suppressor genes. Karyotypic analyses have shown a striking tendency for certain chromosomes to be in parallel excess or deficiency in seminoma and the nonseminomatous germ cell tumors,53 and loss of heterozygosity studies also show similar patterns of allelic imbalance between coexisting seminoma and nonseminoma in the testis.55 These data indicate that seminoma may transform to nonseminomatous tumors. Genetic changes precede the development of an invasive germ cell tumor from IGCNU.19,56,57 Although overrepresentation of gene sequences from the short arm of chromosome 12, mostly in the form of an isochromosome [i(12p)], is consistent in invasive germ cell tumors of adult patients, such overrepresentation is not found in the associated IGCNU.58–60 It is believed that additional 12p sequences are essential for invasive growth by inhibiting apoptosis and thereby permitting survival of invasive tumor cells outside of the microenvironment of the seminiferous tubules.59 On the other hand, marked similarities exist between many of the genetic changes in IGCNU and the associated invasive tumor,55,56 in support of the precursor role of the former. Immunohistochemical study has shown that loss of the cell cycle–dependent kinase inhibitors p18 and p21 accompanies invasive growth,61,62 as does gain of the ubiquitin ligase double minute-2 (mdm-2)61 and increased production of cyclin E.62 Transformation of IGCNU to nonseminomatous tumor apparently may occur at the time of invasion because pure embryonal carcinoma, yolk sac tumor, and choriocarcinoma are associated with IGCNU. Alternatively, these elements may overgrow a small focus of seminoma from which they arose. The common occurrence of seminoma with nonseminomatous elements also supports transformation from invasive seminoma. IGCNU adjacent to seminoma or nonseminomatous tumors shares certain chromosomal abnormalities with the invasive tumor, and these abnormalities differ depending on whether the adjacent tumor is seminomatous or nonseminomatous,63 an observation that supports the occurrence of genetic transformation within the tubules before morphologic change. Additionally, intratubular malignant germ cells that are not morphologically recognizable as embryonal carcinoma cells are reported to show CD30 reactivity, suggesting early-phase differentiation of IGCNU cells to embryonal carcinoma cells within the tubules, followed by rapid extratubular invasion.64 These observations led to a revised model of testicular germ cell tumor histogenesis, based on the tetrahedron model proposed by Srigley and co-workers (Fig. 13-4).52 Fig. 13-4 New model of germ cell tumor histogenesis, based on the tetrahedron model of Srigley and associates.52 In this model, seminoma plays a pivotal role as a precursor for many other forms of germ cell tumor. Note the absence of IGCNU for pediatric teratoma, pediatric yolk sac tumor, dermoid cyst, a select subgroup of nondermoid teratomas and spermatocytic seminoma. IGCNU, Intratubular germ cell neoplasia of the unclassified type; MT, malignant transformation; syntroph, syncytiotrophoblast cells. The histogenesis of testicular germ cell tumors in children is different from that in postpubertal patients. This is reflected in epidemiologic studies that have documented a progressive increase in the incidence of testicular germ cell tumors throughout the twentieth century in postpubertal patients but not in the prepubertal group.65 Unlike postpubertal tumors, the examples in children (virtually confined to two types of neoplasm—yolk sac tumor and teratoma66) lack any consistent association with IGCNU.67–70 Furthermore, teratomas have a diploid DNA content and normal karyotype, unlike those of most postpubertal patients, and the yolk sac tumors lack consistent 12p abnormalities.71–73 Pediatric germ cell tumors thus have a fundamentally different pathogenesis. These observations and others led one group to propose that germ cell tumors have five fundamentally different forms74: type I, represented mostly by the pediatric types; type II, consisting of the usual postpubertal germ cell tumors; type III, consisting only of spermatocytic seminoma; type IV, represented by ovarian dermoid cyst; and type V, consisting of gestational choriocarcinoma. Each has its own unique pattern of gene activation and genomic imprinting. Germ cell tumors of the testis (with the notable exception of spermatocytic seminoma) occur mostly in young males, with the incidence accelerating rapidly after puberty and peaking close to 30 years of age (Fig. 13-5). A small peak occurs in early childhood, but many cases of “testicular cancer” in elderly men correspond to lymphomatous involvement or secondary tumors rather than to germ cell tumors (see Fig. 13-5). Whites have a much higher frequency of testicular germ cell tumors than do others, with the exception of the Maori of New Zealand, who have an incidence comparable to that of white populations.75,76 Native Hawaiians, Native Alaskans, and Native Americans are also at higher risk than other nonwhites.77 Denmark and Switzerland have the highest rates of testicular cancer, with approximately 9 cases per 100,000 males per year, in contrast to the rate in the U.S. white population of approximately 6 per 100,000 males. The rates in Africans and Asians are generally approximately 1 per 100,000 males.78 The incidence of most testicular germ cell tumors increased steadily in the United States during the twentieth century,79,80 and a similar trend was noted in several other countries,81 including Denmark,82–84 Norway,85 England,86–88 Germany,83 Scotland,89 New Zealand,76 Australia,90 Canada,91 Iceland,92 and Japan,87 although, as noted, the rate remained steady in children.65 Numerous studies have demonstrated a higher frequency of testicular germ cell tumors in professional workers or those of higher socioeconomic class in contrast to laborers or those of lower socioeconomic status76,77,93–99 and in those with occupational exposures to fertilizers, phenols, heat, smoke, or fumes100; farm workers94,101; draftsmen94; those in food manufacture and preparation94; leather workers102,103; pesticide applicators104; those exposed to insect repellants101; metal workers105; policemen exposed to handheld radar106; aircraft repairmen107,108; motor vehicle mechanics76; electrical workers, fishermen, paper and printing workers, and foresters93; men of tall stature109; and physicians.76 Other studies have suggested other possible etiologies, including in utero exposure to high estrogen levels,110–113 dietary iron,114 testicular trauma,115 various human leukocyte antigen haplotypes,116–123 a family history of breast cancer,124 early puberty,124,125 early birth order,110 dizygotic twinship,126,127 ichthyosis,128 Marfan syndrome,129 the Li-Fraumeni syndrome,130 and dysplastic nevus syndrome.131 One study found a correlation between testicular cancer and a variant allele of the glutathione-S-transferase π gene.132 Another demonstrated increased risk in the sons of fathers who were wood processors, metal workers, or employed in the food product industry.133 Recently, baldness and a history of severe acne, both associated with high androgen levels at puberty, have been associated with testicular germ cell tumors.134 Marijuana use has been associated with an almost twofold increased risk for testicular nonseminomatous germ cell tumors.135 Most of these associations, however, are weak and fail to account for the general increase in testicular germ cell tumors. It is hypothesized that important causative factors in testicular cancer occur in the antenatal period, with a protective effect in European countries for men born during the World War II era.81,136,137 This protective effect leads to the hypothesis that testicular cancer is causally related to prosperity, probably secondary to its in utero effects. Environmental endocrine-disrupting chemicals have attracted interest as a possible causative factor for germ cell tumors (and the other components of the proposed testicular dysgenesis syndrome).21,57,138,139 Phthalates—chemicals present in plastics—have attracted interest as an environmental endocrine disruptor, but a causative role remains unproved.57,139 The use of alcohol and tobacco, prior vasectomy, and radiation exposure have not been associated with testicular germ cell tumors.75,140–143 Despite the weak correlation of most etiologic factors with testicular germ cell tumors,75,144 four contributing factors are proved: cryptorchidism, prior testicular germ cell tumor, family history of testicular germ cell tumors, and certain disorders of sex development. An estimate of the increased risks associated with these disorders is provided in Table 13-3. Table 13-3 Estimated increased risks for testicular germ cell tumors associated with certain conditions An increased frequency of cryptorchidism, varying from 6.5% to 14.5%, has been found in patients with testicular germ cell tumors,79,111,145–150 which leads to increased risk calculations of 2.5 to 35 times higher among patients with cryptorchidism.146-149,151–158 Such risk does not manifest prior to 20 years of age155 and is probably most accurately assessed as 3.5 to 5.0 times increased over a control population.149,155 If cryptorchidism is unilateral, the noncryptorchid testis is also at increased risk for a testicular germ cell tumor, although at a lower rate than the cryptorchid testis.77,151,153,159–161 Ectopia alone cannot explain the association of cryptorchidism and testicular germ cell tumor, a fact reinforced by the failure of orchidopexy to reduce the risk to that of the general population150,151,162,163 (although experience with orchidopexy in the very young is probably insufficient to rule out an ameliorating effect).77 Early surgical correction, at 6 to 12 months of age, has been recommended as treatment for cryptorchidism164 because the risk reduction is related to age, with a relative risk for developing cancer of 2.23 if orchiopexy is performed before 13 years of age in contrast to 5.4 if performed after 13 years.165 It is possible that cryptorchidism is a marker of patients with a general defect in genitourinary embryogenesis and that cryptorchid testes are “dysgenetic,”166 as supported by abnormalities of the external genitalia or sex chromosomes in some patients with cryptorchidism with germ cell tumors.161,167,168 This has been conceptualized as “testicular dysgenesis syndrome,” a collection of findings including cryptorchidism, infertility, disorders of sex development, IGCNU, and germ cell tumors that may share common etiologic factors.57,169,170 A distinctive array of testicular lesions occurs in the parenchyma adjacent to germ cell tumors, including Leydig cell hyperplasia, microlithiasis, angiopathy, Sertoli cell nodules, tubular atrophy, and multinucleated spermatogonia,171 supporting an underlying developmental problem. Cryptorchidism may disproportionately predispose to seminoma in contrast to nonseminomatous tumors.145,156,172,173 Approximately 2% to 4% of patients with cryptorchidism have IGCNU,174–176 and at least 50% of such patients develop a germ cell tumor within 5 years.177 Giwercman and co-workers178 cite a 2.8% frequency of IGCNU in the cryptorchid population, a fourfold increase over the general population of young men.178 Thus bilateral testicular biopsy at 18 to 20 years of age has been recommended for patients with cryptorchidism.179 A negative result is good evidence of no increased risk,174 although false negative results occur infrequently.180,181 A positive biopsy result should prompt orchiectomy of the affected testis. In testes with extreme atrophy, the biopsy should be directed to sample the region near the rete testis.182 Apart from germ cell tumors, cryptorchid testes have an increased frequency of nodules composed of small tubules lined by immature Sertoli cells, often with central deposits of basement membrane (see Sertoli cell tumor section; Figs. 13-150 to 13-153). These Sertoli cell nodules have been termed Pick adenoma, which is something of a misnomer because they are not true neoplasms.183,184 A second germ cell tumor occurs in the remaining testis of 1% to 5% of patients with a previous germ cell tumor.185–196 The risk of a second germ cell tumor is higher in patients with seminoma, especially in men 30 years old or younger.196 Similarly, a 4.5% to 6.6% frequency of IGCNU occurs in the opposite testis of patients with a germ cell tumor.190,191,197–199 The risk is low in the absence of IGCNU on biopsy because of occasional false negative biopsy results.199 In one study, 5 patients among 1859 (0.3%) who had negative testicular biopsy results from opposite a germ cell tumor developed a second tumor on follow-up.181 Sensitivity is improved if three biopsies of the contralateral testis are performed.200 If the residual testis is either atrophic199,201 or cryptorchid, the risk is even greater,198 with a 23% frequency of IGCNU.197 Unfortunately, almost 50% of cases of contralateral IGCNU would be missed if contralateral biopsies were restricted to patients with atrophy or cryptorchidism.198 One study suggested that atrophy rather than maldescent is the important predictor of contralateral IGCNU.202 Young age at onset of the first tumor202 and bilateral cryptorchidism also appear to be associated with an increased risk for bilateral occurrence.190 It is estimated that biopsy of an atrophic testis opposite a germ cell tumor in a patient younger than 31 years of age will detect IGCNU in one third of patients.202 Approximately 50% of second primary tumors of the testis occur 3 to 5 years after the diagnosis of the initial germ cell tumor,187,203 with a mean of 5.6 to 6.5 years,195,204 but intervals of more than a decade can occur.196,205 Concordant or discordant neoplastic types may occur, with some tendency for concordance of pure seminoma.187 The risk for bilateral tumors in patients with a germ cell tumor is increased approximately fourfold with a positive family history.206,207 Chemotherapy administered for the treatment of the first tumor decreases the risk for a contralateral tumor,189,192 but contralateral tumors may occur even in the absence of IGCNU in biopsy samples of the opposite testis performed at the time of the first germ cell tumor diagnosis.208 An increased frequency of rare alleles of the Ha-ras1 oncogene is seen in patients with testicular germ cell tumors, and these rare alleles are associated with bilaterality and early age of onset.209 In one study, 64% of bilateral germ cell tumors had KIT mutations in contrast to 6% of unilateral tumors, suggesting a causative role for KIT mutations occurring early in embryonal development in patients with bilateral tumors.210 First-degree male relatives of patients with germ cell tumor of the testis have a 3 to 10 times greater risk for a testicular germ cell tumor than the general population.211–214 The risk is highest for brothers (10×), intermediate for sons (6×), and lowest for fathers (4×).215 Also, a family history of testicular germ cell tumor is associated with an 8% to 14% frequency of bilaterality206,212,215 in contrast to the 1% to 5% frequency in the general population of patients.185–188,196 The occurrence of an unexpected number of testicular germ cell tumors in the relatives of children with soft tissue sarcoma has raised the question of whether testicular germ cell tumors may represent part of the spectrum of the Li-Fraumeni cancer syndrome.216,217 It appears, however, that neither germline nor somatic p53 mutations occur in these cases.218,219 Immunohistochemical demonstration of p53 protein220,221 therefore indicates overexpression of nonmutated protein. Segregation analysis of data regarding familial cases has suggested the presence of a major gene that conveys risk in a recessive model.222 Genetic linkage analysis has implicated a susceptibility gene localized to Xq27223 in one study, but this was not confirmed in a second.224 Patients with some disorders of sex development are at increased risk for germ cell tumors. Patients with gonadal dysgenesis in the presence of a Y chromosome, including patients with pure 46,XY gonadal dysgenesis (Swyer syndrome), mixed gonadal dysgenesis, and dysgenetic male pseudohermaphroditism, commonly develop gonadal germ cell tumors.225–228 Approximately 25% to 30% of such patients develop gonadoblastoma,225,226,228 and this may serve as the precursor lesion for the development of an invasive germ cell tumor (see Gonadoblastoma section). This risk is modified by the degree of gonadal ectopy and the particular syndrome.228a IGCNU is also present in approximately 8% of children and adolescents with gonadal dysgenesis.229 Because an invasive tumor may develop in childhood, gonadectomy is indicated for those with high-risk conditions as soon as the diagnosis is established. Male pseudohermaphrodites with androgen insensitivity syndrome develop a malignant germ cell tumor in 5% to 10% of cases overall,230–234 but the risk is considerably greater (approximately 50%) for patients with partial androgen insensitivity and nonscrotal testes.228a These patients have been shown to have various mutations in the androgen receptor gene.235 The tumor usually develops after puberty, and this may permit delayed gonadectomy until full feminization has occurred, although this remains controversial because of an occasional case of invasive germ cell tumor developing at an early age. Delay of prophylactic gonadectomy beyond the early postpubescent period in patients with androgen insensitivity syndrome is risky,230 with a 22% frequency of malignant germ cell tumor in these patients beyond 30 years of age.236 Not all testicular masses in patients with the androgen insensitivity syndrome are germ cell tumors; these patients commonly develop hamartomatous nodules composed of Sertoli cell–lined tubules with intervening clusters of Leydig cells in the interstitium, as well as pure Sertoli cell adenomas225,232,233 (see Sertoli cell tumor section) and occasional juvenile granulosa cell tumors.227 Additionally, a single case has been reported of IGCNU in a male pseudohermaphrodite with a nonsense mutation in the gene for steroidogenic acute regulatory protein.237 Patients with infertility have less than a 1% frequency of testicular germ cell tumor.27,176,197,238 The relative risk in patients with infertility is much lower than that associated with cryptorchidism or a history of contralateral germ cell tumor.239 It is not clear, however, if infertility is a risk factor for germ cell tumor that is independent of cryptorchidism or gonadal dysgenesis.240 Impaired semen quality, hypospadias, cryptorchidism, and germ cell tumors are all components of the testicular dysgenesis syndrome and are interrelated.138 Semen quality may be a highly sensitive marker for environmental exposures that are also related to germ cell tumor incidence.138 Testicular microlithiasis, seen ultrasonographically as hyperechogenic foci without shadowing,241 is observed in a small percentage of patients evaluated by testicular ultrasound. Rare cases of germ cell tumor have been reported in patients, including teenage boys, being followed for testicular microlithiasis.242 It is unusual for a patient with testicular microlithiasis, but without other risk factors, to have IGCNU on biopsy, so biopsy is recommended only if other risk factors such as infertility, cryptorchidism, or contralateral tumor exist.241 Grossly, the testis with IGCNU may be unremarkable or appear atrophic and fibrotic. Microscopically, intratubular germ cell neoplasia consists of a proliferation of malignant germ cells that may be a specific neoplastic type, such as intratubular embryonal carcinoma or intratubular spermatocytic seminoma, or may consist of undifferentiated germ cells resembling atypical gonocytes. The atypical gonocyte-like form of intratubular germ cell neoplasia is typically confined to the basilar aspect of the seminiferous tubules and is associated with all types of postpubertal germ cell tumors, except for spermatocytic seminoma, dermoid and epidermoid cyst, and rare benign teratomas.22 IGCNU cells have enlarged, hyperchromatic nuclei, often with one or two prominent nucleoli, thickened nuclear membranes, and clear cytoplasm (see Fig. 13-3). The median nuclear diameter is 9.7 µm, in contrast to a median nuclear diameter in spermatogonia of 6.5 µm.243 Spermatogenesis in the affected tubules is usually decreased or absent, and the tubules may have a thickened peritubular basement membrane. Sertoli cells are often displaced luminally (Fig. 13-3). The distribution of IGCNU is characteristically patchy, and adjacent profiles of seminiferous tubules may appear unremarkable, with intact spermatogenesis (Fig. 13-6). The patchy distribution has implications for biopsy diagnosis, because a small-volume sample may result in a false-negative diagnosis.244 Leydig cell hyperplasia may occur in the interstitium. IGCNU often spreads into the rete testis in a pagetoid fashion, intermixing with nonneoplastic epithelium (Fig. 13-7).245 The strong similarity of the cells of seminoma and IGCNU suggests that IGCNU could be termed intratubular seminoma; however, this term may falsely connote that IGCNU is the precursor lesion for only seminoma rather than for virtually all postpubertal germ cell tumors. By convention, the term intratubular seminoma is reserved for those proliferations of IGCNU-like cells that fill and distend seminiferous tubules (Fig. 13-8), although such lesions may fundamentally simply be a more advanced stage of IGCNU.246 Fig. 13-8 Distention of seminiferous tubules by seminoma cells is referred to as intratubular seminoma. Glycogen is present in the cytoplasm of 98% of cases of IGCNU (Fig. 13-9),247 and its demonstration is diagnostically helpful but nonspecific because nonneoplastic spermatogonia and Sertoli cells also may contain glycogen.248 The current best immunohistochemical marker for IGCNU is OCT4, a nuclear transcription factor with a key role in maintaining pluripotency in embryonic stem cells. OCT4 is uniformly sensitive for IGCNU (Fig. 13-10, A).36,37,249,250 It has also been reported in germ cells with delayed maturation in gonadal dysgenesis,251 Down syndrome,252 and undervirilization syndromes253 and has features different from those of IGCNU. Other IGCNU markers (CD117 and PLAP) are also positive in germ cells with delayed maturation.254 PLAP is also a good marker for IGCNU. It highlights the cytoplasmic membranes of most cases (reported sensitivity, 83% to 100%, see Fig. 13-10, B).32,248,255,256 Only rarely (<1%) are isolated nonneoplastic spermatocytes (which are unlikely to be confused with IGCNU) PLAP positive, with spermatogonia being PLAP negative.257 Immunohistochemical staining for NANOG, a regulatory factor upstream to OCT4, similarly marks IGCNU.38 IGCNU has been found to react with monoclonal antibodies M2A (D2-40/gp36/podoplanin)29,256,258,259; 43-9F256,260; TRA-1-6030; HB5, HF2, and HE11261; and with antibodies directed against glutathione-S-transferase isoenzyme π,34 CD117 (c-kit; see Fig. 13-10, C),210,250,262 angiotensin-converting enzyme,263 p53,264 growth differentiation factor 3 (GDF3),250 stem cell factor,39 and Krüppel-like factor 4.265 It is negative for the RNA-binding motif protein, in contrast to nonneoplastic germ cells.266 IGCNU is SOX17 positive and SOX2 negative, but SOX17 also marks nonneoplastic germ cells, a problem we have also encountered with CD117.41,42,267 Although only occasionally seen, intratubular embryonal carcinoma is SOX17 negative and SOX2 positive.41 The positive reactions for PLAP, OCT4, CD117, and TRA-1-60 support the concept that IGCNU resembles fetal gonocytes.30,32,268 By electron microscopy, IGCNU has evenly dispersed chromatin, intricate nucleoli, and sparse cytoplasmic organelles with prominent glycogen deposits. Occasional rudimentary intercellular junctions may be identified.27,269–272 These features are essentially the same as those of seminoma.273 The DNA content of IGCNU is similar to that of seminoma—usually in the triploid and hypotetraploid range.43,274 Some of the genetic features of IGCNU have been previously mentioned (see Histogenesis section). IGCNU should be distinguished from specific forms of intratubular germ cell neoplasia. Intratubular seminoma fills and distends the tubules, whereas IGCNU is restricted to the basilar area, although the cells are histologically identical. Atypical germ cells that do not resemble IGCNU cells also may occur in seminiferous tubules. These cells may have large nuclei or be multinucleated. They lack the cytoplasmic clarity and nucleolar prominence of IGCNU cells and do not stain for the usual IGCNU markers. Although they may indicate a perturbation in testicular development, as evidenced by increased frequency in boys with cryptorchidism275 and location adjacent to germ cell tumor,171 their significance is not clear, unlike in IGCNU. As mentioned, germ cells with delayed maturation may be confused with IGCNU (Fig. 13-11). These cells are seen in young children who have conditions that place them at increased risk for germ cell tumors, including gonadal dysgenesis, Down syndrome, and undervirilization syndromes.252,253,276 Their full clinical significance is not yet clear, meaning it is not known how frequently they will progress to an invasive germ cell tumor, although it seems likely that these cells do play a precursor role in some cases. It is important to distinguish them from IGCNU because IGCNU will progress in virtually all cases. Germ cells with delayed maturation are seen in prepubertal patients throughout the testis and are more or less randomly placed in the seminiferous tubules, including centrally. IGCNU, on the other hand, is seen almost exclusively in postpubertal patients unless the patient has a disorder of sex development, tends to be patchily distributed in the parenchyma, and occupies a basilar location within seminiferous tubules. The practical importance of IGCNU is its progression to an invasive germ cell tumor (either seminomatous or nonseminomatous) in approximately 50% of cases within 5 years after identification.277 Also, only a small fraction of patients remain free of an invasive tumor by 7 or 8 years of follow-up (Fig. 13-12),278 although some patients may not develop a tumor for more than 15 years.178 Furthermore, no case of spontaneous regression of typical IGCNU has been documented.178 IGCNU is identified with increased frequency in patients with cryptorchidism, a previous history of a testicular germ cell tumor, gonadal dysgenesis, androgen insensitivity syndrome, and infertility.* IGCNU is also identified in the residual seminiferous tubules of virtually every postpubertal patient with an invasive testicular germ cell tumor, with the exceptions of spermatocytic seminoma and dermoid and epidermoid cysts.177,247,289–291 The pediatric germ cell tumors, as mentioned previously (see Histogenesis section), lack association with IGCNU. In our opinion, many reported cases in children represent reactive enlargement of nonneoplastic germ cells induced by a mass lesion (Fig. 13-13), as described by Hawkins and Hicks,292 atypical but nonneoplastic germ cells of unknown significance, or examples of delayed maturation of germ cells. Testicular biopsies are a sensitive method for detecting IGCNU. Fixatives such as Bouin, B-5, and Stieve enhance cytologic detail and may permit easier detection of IGCNU in contrast to formalin fixation. Formalin, Bouin, and Stieve fixatives permit immunohistochemical detection of PLAP, whereas Cleland fluid yields inconsistent results.256 Fixation with fixatives other than formalin may result in weaker immunohistochemical staining, so formalin may be preferred if immunohistochemical staining is expected.293 Berthelsen and Skakkebaek294 concluded that one or two 3-mm biopsy specimens of a testis harboring IGCNU will detect virtually every case, although rare false-negative results do occur (0.3% in one study of 1859 biopsy samples that were initially interpreted as negative181). Another study supported three biopsy samples, each 5 mm in length, as optimal.200 In cases of severe atrophy, with obliteration of many tubules, it may be necessary to sample the region near the hilum, where IGCNU is more frequently preserved within the epithelium of the rete testis.182 Recently, some authors have considered OCT4 immunohistochemistry mandatory for the evaluation of testis biopsy samples because OCT4 staining identifies IGCNU in approximately 20% more cases than hematoxylin and eosin (H&E) staining alone.244,293 Potential populations for screening biopsies include patients with cryptorchidism (2% to 4% positivity for IGCNU), a prior testicular germ cell tumor (5% positivity), somatosexual ambiguity (25% positivity), and, less strongly, oligospermic infertility (0 to 1% positivity).176,178,243 IGCNU in a biopsy sample from a patient with retroperitoneal germ cell tumor probably indicates regression of a prior invasive tumor that had metastasized to the retroperitoneum.295,296 Therefore a patient with presumed primary germ cell tumor of the retroperitoneum may benefit from testicular biopsy.178 Because of the high rate of progression of IGCNU to invasive testicular germ cell tumor, these patients should receive appropriate ablative treatment. Unilateral IGCNU is usually managed by orchiectomy, and bilateral IGCNU may be treated by bilateral orchiectomy or radiation. IGCNU is very radiosensitive; therefore low doses are used, permitting preservation of androgen function. Chemotherapy, often given to patients with metastatic disease from a contralateral testicular tumor, may ablate IGCNU in the residual testis but is not a consistently effective means of therapy.243,297–300 Seminoma is the most common form of pure testicular germ cell tumor, and pure seminoma accounts for approximately 50% of all cases of testicular germ cell tumor, although its relative proportion may be declining. In one study, the percentage of seminomas decreased from 52% to 43% over the 20-year study period.301–304 It occurs in patients with an average age of 40 years,301 which is approximately 10 years older than those with nonseminomatous germ cell tumor; African-Americans may have an earlier age of onset.47,301,305 Seminoma is extremely rare before puberty, but occasional cases in patients younger than 20 years of age are seen.306–309 Most patients present with a painless testicular mass, but also may have a dull, aching sensation. Up to 11% have normal-sized or atrophic testes.310 Occasional patients (2% to 3%) present with symptoms of metastases, usually back pain resulting from retroperitoneal involvement, but gastrointestinal bleeding, bone pain, central nervous system dysfunction, dyspnea and cough, and other symptoms may rarely be the symptoms the patient initially reports.11 Gynecomastia may occur as a result of elevation of serum hCG because of intermingled syncytiotrophoblast elements in seminoma and is rarely a presenting feature311; also, very rarely, a paraendocrine form of exophthalmos can be a presenting symptom,312,313 as can paraneoplastic hypercalcemia,314 hemolytic anemia,315 and limbic encephalopathy.316 Although symptoms of metastases are unusual presenting symptoms in patients with seminoma, approximately 30% have metastases at the time of diagnosis.301 However, the percentage of patients with germ cell tumors presenting with stage I disease is increasing (from 57% to 75% in a recent 20-year study).304 Patients with seminoma usually lack serum AFP and hCG elevations that occur commonly in patients with nonseminomatous germ cell tumors. AFP levels should be normal, although concomitant liver disease (including seminomatous metastases to the liver) may cause modest AFP elevation,317 and “borderline” elevated AFP levels have been seen in some cases without evidence of nonseminomatous elements.318 Most oncologists regard significant AFP elevation in a patient with apparently pure testicular seminoma as evidence of nonseminomatous elements and treat accordingly. Approximately 10% to 20% of patients with clinical stage I “pure” testicular seminoma have elevated serum hCG, and 25% or more with advanced seminoma have hCG elevations.319 At initial diagnosis, 7% to 25% of patients with seminoma have elevated hCG.320–326 If blood is sampled from the testicular vein, 80% to 85% of patients have elevated hCG.327,328 Such elevation reflects the presence of intermingled syncytiotrophoblast elements in these tumors, and the elevations are generally modest. Elevations of serum hCG exceeding 40 IU/L have been correlated with a worse prognosis, although this is controversial.320,329,330 Peripheral venous elevations of hCG have been correlated with larger tumors, perhaps explaining the relationship of elevated hCG with adverse prognostic features.328 Elevation of serum levels of lactate dehydrogenase, PLAP, and neuron-specific enolase (NSE) also may occur in patients with seminoma. Such elevations, however, are neither specific nor especially sensitive, which limits their clinical utility.319,330–334 Grossly, seminoma is usually cream to tan and often multinodular (Fig. 13-14), with occasional yellow foci of necrosis. Infrequently, necrosis is extensive. In some cases the tumor is diffuse, fleshy, and encephaloid, similar to testicular lymphoma (Fig. 13-15). In contrast to lymphoma, however, only approximately 10% of seminomas extend into paratesticular structures.335 Intraparenchymal hemorrhage may cause red discoloration.26 The cut surface of seminoma usually bulges from the surrounding parenchyma (see Fig. 13-14). Punctate foci of hemorrhage often correspond to intermingled foci of syncytiotrophoblast elements.336 A fibrous consistency is uncommon but results when prominent fibrous septa develop in the tumor or if there is extensive intratumoral sclerosis. Microscopically, seminoma usually is arranged in a diffuse, sheetlike pattern interrupted by branching, fibrous septa containing an inflammatory infiltrate (Fig. 13-16) consisting chiefly of lymphocytes but often containing plasma cells and sometimes eosinophils. Distinct nodules of seminoma may be apparent, sometimes with confluent growth imparting a lobulated pattern. In some cases, a prominent cordlike arrangement of cells is present (Fig. 13-17), often at the periphery of nodules showing a sheetlike pattern. Foci of intertubular growth may be seen, with preservation of seminiferous tubules; this pattern is usually most apparent at the periphery of neoplastic nodules and may be associated with rete testis invasion and aggressive behavior.337 In rare seminomas, an intertubular pattern may predominate (Fig. 13-18), with well-preserved seminiferous tubules even in the central portions of the neoplasm. Such cases are prone to being overlooked because they do not destroy the seminiferous tubules and they often do not form a discrete mass.338 The presence of a lymphocytic infiltrate may be a clue to intertubular seminoma (see Fig. 13-18). This growth pattern is much more common, however, in testicular lymphoma (see Lymphoma section). With time, many seminomas develop foci of scarring. Hyalinized deposits of collagen may separate the neoplastic cells into small nests resembling solid pseudotubules (Fig. 13-19). Extensive collagen deposits may result in broad scars with only a few scattered neoplastic cells. Calcification and even ossification may rarely occur in hyalinized fibrous trabeculae of seminoma.339 Rarely, seminoma may show a distinctly tubular pattern in which a palisade-like arrangement of neoplastic cells occurs at the periphery of tubule-like structures that may contain loosely cohesive neoplastic cells in their “lumina” (Fig. 13-20).340–343 Seminoma also may develop intercellular edema with separation of neoplastic cells and formation of microcystic spaces (Figs. 13-21 and 13-22). These spaces are generally, but not always, irregular in outline and frequently contain visible edema fluid and intracystic exfoliated neoplastic cells344 which contrast with the “cleaner,” more round and regular microcystic spaces commonly identified in yolk sac tumor (see Yolk sac tumor section). Foci of coagulative necrosis are present in approximately one half of seminomas and may be extensive in a minority of cases.310 Fig. 13-16 Sheetlike pattern of seminoma is interrupted by branching, fibrous septa containing lymphocytes. A lymphoid infiltrate is a virtually constant feature of seminoma and is usually most evident in perivascular areas and around fibrous trabeculae, which also contain many capillaries (Fig. 13-23). Lymphocytes are also frequently intermingled with the seminoma cells elsewhere. A florid lymphoid reaction, with formation of germinal centers, occurs in a minority of cases, but most of the lymphocytes in seminomas are T cells.345–351 Ultrastructural studies have demonstrated a cytolytic effect of the lymphocytes on the seminoma cells,350 correlating with the observation of some investigators of a better prognosis in cases associated with a prominent lymphocytic reaction.352 A variable granulomatous reaction occurs in up to 50% of seminomas. In most cases, this reaction consists of small clusters of epithelioid histiocytes scattered among neoplastic cells (see Fig. 13-23); Langhans-type giant cells and other multinucleated giant cells may be present. Intratubular collections of epithelioid histiocytes also may be seen. Rarely, the granulomatous reaction can be extensive and virtually efface the neoplasm (Fig. 13-24); in such cases, it may be difficult to distinguish a florid granulomatous reaction in a seminoma from granulomatous orchitis, and careful search for residual seminoma and IGCNU is indicated. The cells of seminoma are generally clear to lightly eosinophilic, measuring 15 to 25 µm in diameter. The nuclei are uniform, round to oval, and usually central or slightly eccentric, with finely granular chromatin and one or two prominent nucleoli (Fig. 13-25). Often some nuclei have a relatively flat edge that has been described as “squared-off,” lending the nuclei a “boxy” appearance. The nuclear membranes are irregularly thickened. The cell borders are well defined in adequately fixed specimens (Fig. 13-25). Abundant cytoplasm separates the nuclei so that overlapping nuclei are not seen. Occasionally, seminoma displays foci of increased cellular atypia with less well-defined cytoplasmic boundaries, darker cytoplasm, and enlarged, crowded nuclei (Fig. 13-26). These changes may impart a plasmacytoid appearance to the tumor cells (Fig. 13-27). Such changes may be seen in association with early necrosis and contain pyknotic nuclear fragments. Such isolated foci, in the absence of distinct epithelial differentiation, should not militate against a diagnosis of seminoma. Rarely, seminoma cells develop intracytoplasmic vacuoles that cause a signet ring cell morphology (Fig. 13-28).353 This may be a focal finding in an otherwise normal-appearing tumor. We also have seen prominent aggregates of glycogen create a signet ring cell appearance. Fig. 13-26 Seminoma with increased nuclear pleomorphism and crowding and denser cytoplasm than usual. Mitotic figures in seminoma are prominent, and, in the past, when present in sufficient numbers, were considered evidence of “anaplastic seminoma.”152 Now it is clear that the practice of using an average of three mitoses per high-power field identified far too many seminomas as “anaplastic,”354 and, more importantly, evidence shows that high mitotic rate seminoma behaves no differently than seminoma with a lower mitotic rate.355,356 Furthermore, no immunohistochemical difference exists between typical and “anaplastic” seminomas.356 Use of the term anaplastic seminoma is therefore discouraged. Although some seminomas behave more aggressively than most seminomas, it remains unclear whether such cases can be identified prospectively. Tickoo and associates357 described cases of “seminoma with atypia” based on nuclear pleomorphism and crowding, paucity of lymphocytes, and darker staining cytoplasm. In their experience, such tumors were more likely to present at advanced clinical stage and express CD30 and lose CD117 expression. It remains unclear, however, whether such cases should receive a different therapy. Syncytiotrophoblastic cells are present in 10% to 20% of seminomas.50 The morphology of these cells is variable, ranging from typical syncytiotrophoblastic cells having cytoplasmic lacunae and multinucleation (Fig. 13-29) to large, mononucleated or binucleated cells that may not be easily distinguished from the background of seminoma cells but are highlighted by immunostains directed against hCG.358 Intermediate between these extremes are cells containing multiple nuclei in a “mulberry” pattern. Syncytiotrophoblastic cells are often located close to capillaries, and microhemorrhages may be seen in these foci. Unlike in choriocarcinoma, the syncytiotrophoblastic cells are not intermingled with a mononucleated trophoblastic cell component but are more or less randomly admixed as single cells or small clusters with seminoma cells. With hCG immunohistochemistry, intratubular trophoblast can be detected in a substantial fraction of seminoma cases, indicating that differentiation toward trophoblastic elements may occur prior to invasion.359 Glycogen is usually prominent in seminoma (Fig. 13-30), and most cases show immunoreactivity for PLAP, generally in a peripheral, membranous staining pattern (Fig. 13-31, A); cytoplasmic staining also may be identified.33,248,360,361 Angiotensin-converting enzyme was demonstrated in 100% of 91 seminomas in one study.263 Reactivity for CD117 occurs in most seminomas, again with a cytoplasmic membrane pattern similar to PLAP (see Fig. 13-31, B).362
Neoplasms of the testis
Staging
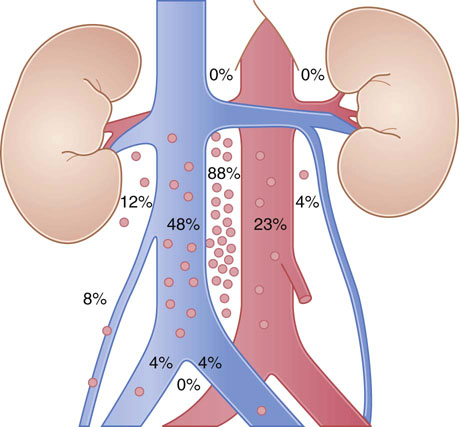
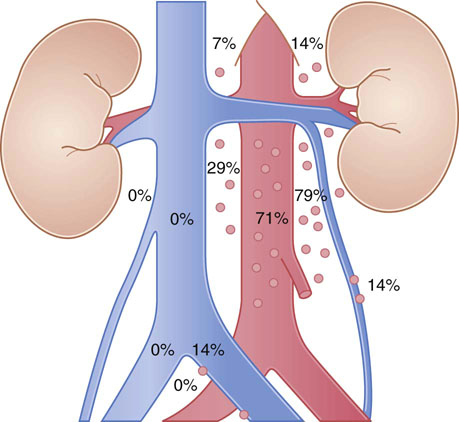
Patterns of metastasis
Gross examination
Germ cell tumors
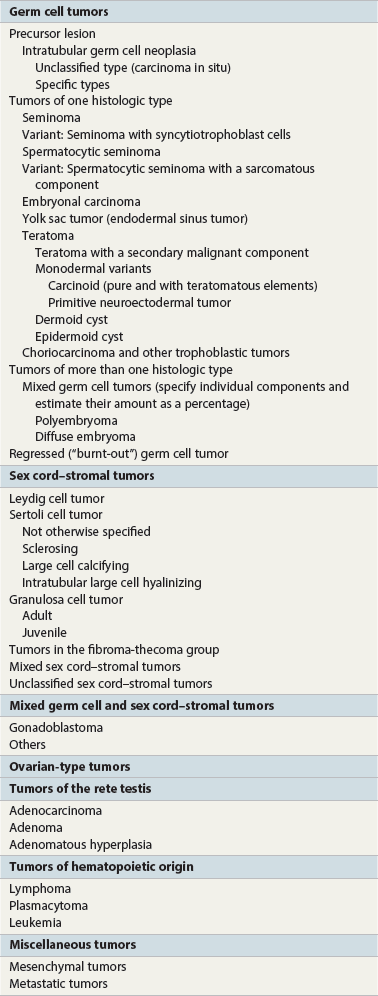
Classification
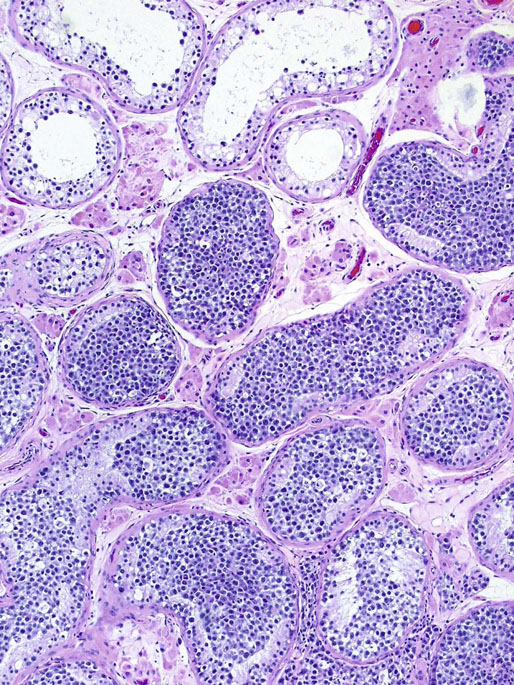
Histogenesis
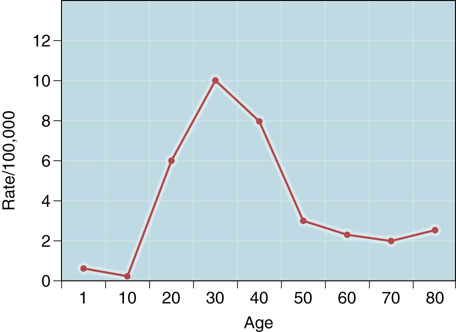
Epidemiology
Condition
Estimated increased risk
Cryptorchidism149,155
3.5-5×
Prior testicular germ cell tumor1,176,190,197,198,198
5-10×
Family history (first-degree male relative)211–214
3-10×
Gonadal dysgenesis with a Y chromosome1,225,226
50×*
Androgen insensitivity syndrome1,230–233
15×
Cryptorchidism
Prior testicular germ cell tumor
Family history
Disorders of sex development
Infertility
Other associations
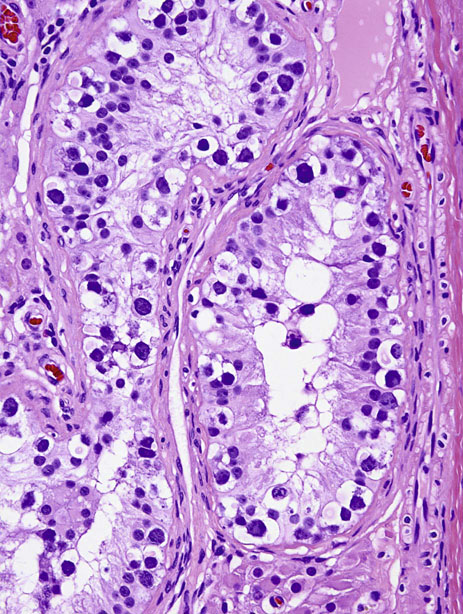
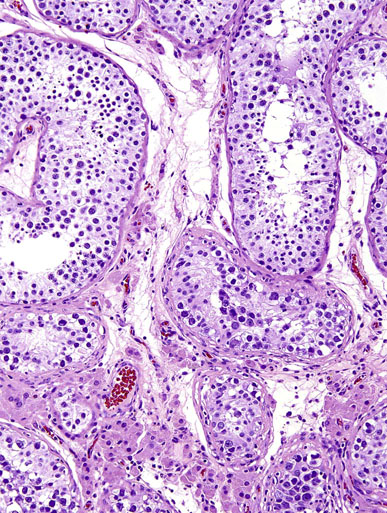
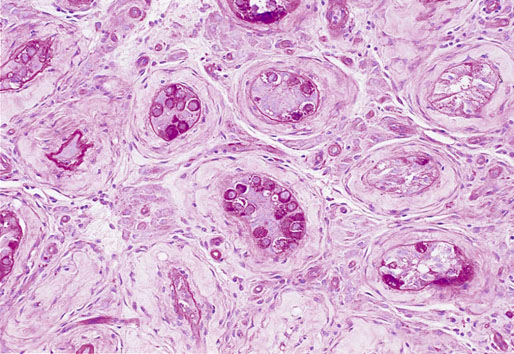
Intratubular germ cell neoplasia
Special studies
Differential diagnosis
Prognosis
Biopsy diagnosis
Treatment
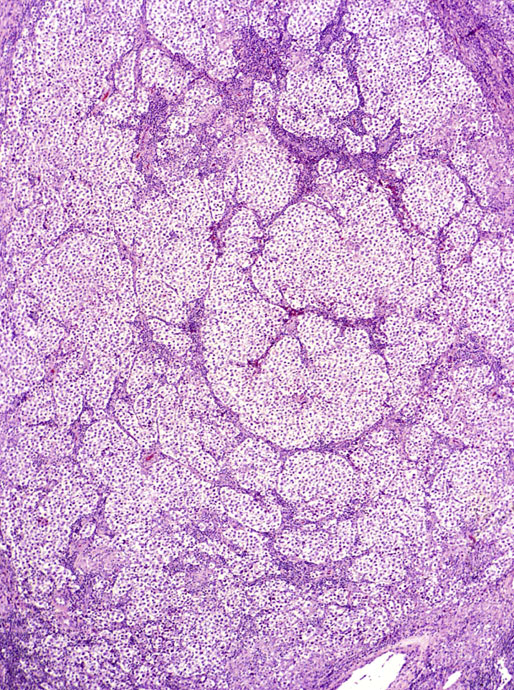
Seminoma
Clinical features
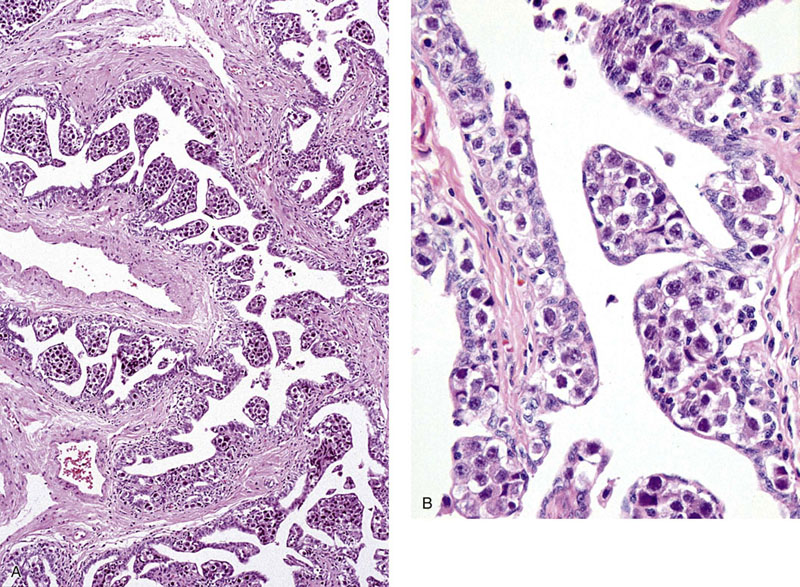
Pathologic findings
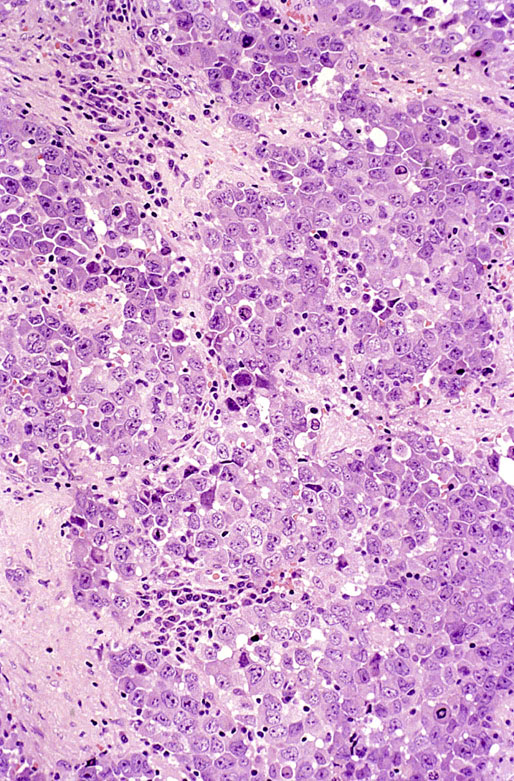
Seminoma with syncytiotrophoblastic cells
Special studies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neoplasms of the testis