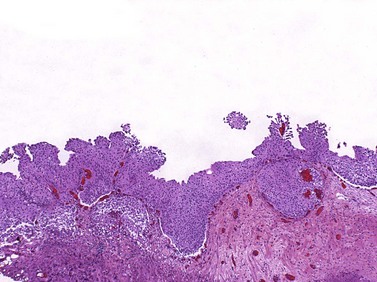
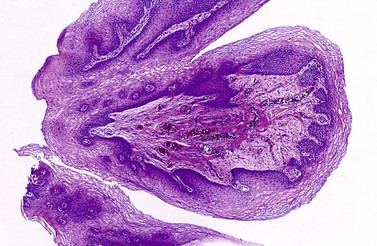
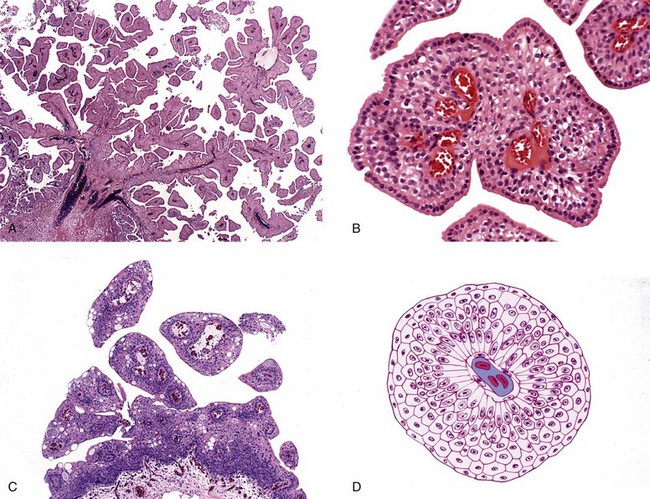
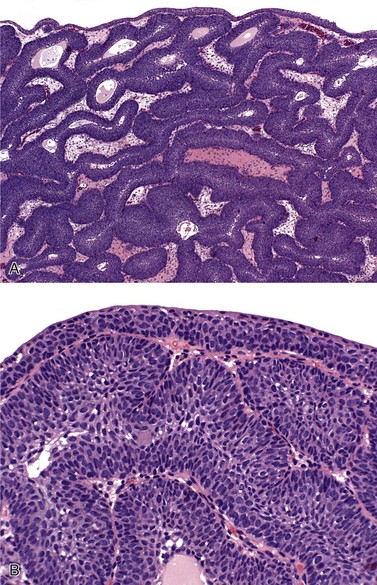
Chapter 6 Benign urothelial (transitional cell) neoplasms Flat urothelial hyperplasia (simple hyperplasia) Urothelial dysplasia (low-grade intraurothelial neoplasia) Urothelial carcinoma in situ (high-grade intraurothelial neoplasia) Therapy-induced changes in the urothelium and mimics of urothelial flat neoplasia Urothelial (transitional cell) carcinoma Neural and neuroendocrine tumors Large cell neuroendocrine carcinoma Paraganglioma (pheochromocytoma) Primitive neuroectodermal tumor Metastatic tumors and secondary extension Carcinoma of the urinary bladder is the fourth most common malignancy in men, accounting for an estimated 72,570 new cases and 15,210 cancer deaths in the United States in 2013.1–4 Bladder cancer is morphologically heterogeneous; more than 90% of bladder cancer cases are urothelial (transitional cell) carcinoma, whereas primary squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and other tumors are less common.2 The classification system of urinary bladder neoplasia used in this chapter has been modified according to the most recent World Health Organization (WHO) classification of tumors of the urinary system.5 Urothelial papilloma is a benign exophytic urothelial neoplasm that typically occurs in patients younger than 50 years of age.6 The male-to-female ratio is 2 : 1. The most common symptom is hematuria.6 Most tumors are located near the ureteric orifices. Urothelial papilloma may recur, but recurrent papilloma does not progress.6,7 The diagnostic criteria for urothelial papilloma in the 2004 WHO classification5 are identical to those defined in the 1973 WHO classification.8 Using the restrictive diagnostic criteria recommended by the WHO, urothelial papilloma represents less than 1% of papillary urothelial neoplasms.6,9–12 Urothelial papilloma is composed of a delicate fibrovascular core covered by cytologically and architecturally normal urothelium with no more than seven layers of cells (Fig. 6-1). The superficial cells are often prominent and may have vacuolization of the cytoplasm, eosinophilic syncytial morphology, or apocrine-like morphology or may demonstrate mucinous metaplasia.10,12 Mitotic figures are absent to rare and, if present, are located in the basal cell layer. The stroma may show edema or inflammatory cells. Rare cases show dilated lymphatics within the fibrovascular fronds. Occasionally, foamy histiocytes accumulate within the fibrovascular stalks. Secondary budding of small fronds from larger simple primary papillary fronds is commonly observed. Fig. 6-1 Urothelial papilloma is a papillary lesion composed of delicate fibrovascular cores lined by cytologically and architecturally normal urothelium less than seven cell layers thick. A, Low-power view of urothelial papilloma. B, High-power view of the same tumor as in A. C, Another example of urothelial papilloma. D, Schematic diagram of urothelial papilloma. (From Cheng L, Darson M, Cheville JC, et al. Urothelial papilloma of the bladder: clinical and biological implications. Cancer 1999;86:2098-2101, with permission.) Papilloma is a diploid tumor with a low proliferation rate, undetectable or very limited p53 accumulation, and frequent fibroblast growth factor receptor 3 (FGFR3) mutation (75% of cases).13 Cytokeratin 20 (CK20) expression is limited to the superficial (umbrella) cells.12,14 The designation diffuse papillomatosis is applicable when the mucosa is extensively involved by multiple small, delicate papillary processes, creating a velvety cystoscopic appearance.10,12,15 These papillary structures are covered by normal urothelium with no cytologic atypia (Fig. 6-2). The malignant potential of this lesion is unclear. Inverted papilloma is usually found in men in the sixth or seventh decade of life.16–25 The male-to-female ratio is 7 : 1.16 Hematuria and obstructive symptoms are the most common symptoms at presentation. The majority of inverted papillomas develop in the region of the trigone and bladder neck (Fig. 6-3). Some cases may be multifocal.16,26 The incidence of multiplicity ranges from 1.3% to 4.4%.16,18 A significant number of patients have a history of smoking, suggesting a possible link between tobacco use and inverted papilloma.16 Fig. 6-3 Distribution of inverted papillomas in the urinary tract. (From Sung MT, MacLennan GT, Lopez-Beltran A, et al. Natural history of urothelial inverted papilloma. Cancer 2006;107:2622-2262, with permission.) Histologically, inverted papilloma shows an inverted growth pattern, usually composed of anastomosing islands and trabeculae of histologically and cytologically normal urothelial cells invaginating from the surface urothelium into the subjacent lamina propria but not into the muscularis propria (Fig. 6-4). Although the term inverted papilloma was initially introduced in 1963 by Potts and Hirst to describe this architecturally distinctive urothelial neoplasm,27 the Viennese urologist Paschkis had previously reported four morphologically identical urothelial tumors in 1927 under the name adenomatoid polyp.28 Kunze and colleagues proposed the subdivision of inverted papilloma into two morphologically distinct variants, trabecular and glandular.17 By their criteria, the trabecular variant is composed of anastomosing cords and trabeculae of urothelial cells invaginating the lamina propria at various angles. These invaginating structures demonstrate mature urothelium centrally, with darker and palisading basal cells peripherally, usually surrounded by fibrotic stroma without marked inflammation. The glandular variant is composed of nests of urothelium with either pseudoglandular spaces lined by mature urothelium or even true glandular elements containing mucicarminophilic secretions and mucous-secreting cells. The glandular variant, as proposed by these investigators, has considerable morphologic overlap with florid cystitis glandularis and is not widely accepted as a diagnostic entity. Fig. 6-4 Inverted urothelial papilloma. A, The low magnification demonstrates a distinct downward growth pattern of a typical inverted papilloma composed of intact surface-lining urothelium and underlying thin anastomosing trabeculae of urothelium in the lamina propria. B, At higher magnification, the trabeculae are lined by uniform urothelial cells without cytologic atypia. Within the spectrum of findings in inverted papilloma, vacuolization and foamy xanthomatous cytoplasmic changes may be seen. These “clear cells” may be concentrated within distinct regions of the tumor, but more frequently are diffusely intermingled with usual inverted papilloma cells. Foci of nonkeratinizing squamous metaplasia and neuroendocrine differentiation have been reported.26 Mitotic figures are either absent or rare. Some cases may demonstrate focal minor cytologic atypia that is likely degenerative and has no clinical significance.29 Inverted papilloma may coexist with carcinoma, and it is most important to differentiate inverted papilloma from urothelial carcinoma with inverted growth pattern. This distinction may be difficult, especially in limited biopsy specimens or when interpretation is confounded by crush artifact.29 Inverted papilloma usually exhibits orderly maturation of invaginated trabeculae and cords, composed of spindling and peripherally palisading cells. In contrast, urothelial carcinoma with inverted growth pattern often has thick and irregular tumor columns with transition to more solid nests. Additionally, the presence of an exophytic papillary component and unequivocal tumor invasion in the lamina propria or muscularis propria justify a diagnosis of inverted urothelial carcinoma. Marked cytologic atypia, including nuclear pleomorphism, nucleolar prominence, and abundant mitotic activity further supports a diagnosis of malignancy. Several investigators have voiced concern regarding the malignant potential of inverted papilloma based on the subsequent development of urothelial carcinoma, but the great majority of patients with this complication have a history of a previous or concurrent urothelial carcinoma.20,30–33 In a large series of 75 patients, all but one patient had an uneventful course without either tumor recurrence or progression to urothelial malignancy during a mean follow-up of 68 months.16 Consequently, transurethral resection of inverted papilloma is adequate treatment, and surveillance protocols as rigorous as those employed in the management of urothelial carcinoma seem unnecessary for this benign entity. It has been well documented by the finding of nonrandom inactivation of X chromosomes that inverted papilloma is a clonal neoplasm that arises from a single progenitor cell.34 Sung and associates,34 Baud and associates,35 Keen and Knowles,36 Cheng and colleagues,37 Louhelainen and associates,38 Paterson and colleagues,39 and Jones and colleagues40 studied the status of loss of heterozygosity (LOH) in inverted papilloma using microsatellite markers that are commonly altered in urothelial carcinoma. The incidence of LOH in inverted papilloma is low (8% to 10%) and contrasts to the high frequency of LOH (29% to 80%) in urothelial carcinoma and papillary urothelial neoplasm of low malignant potential.* The low frequency of allelic loss in inverted papilloma is similar to that of normal urothelium.43 Lott and colleagues44 evaluated mutations of FGFR3 and TP53 genes by DNA sequencing in 20 patients with inverted papilloma of the urinary tract.44 Point mutations of the FGFR3 gene were identified in 45% (9 of 20) of inverted papillomas, with 4 exhibiting mutations at multiple exons. Seven patients had exon 7 mutations of FGFR3 gene, containing R248C, S249T, L259L, P260P, and V266M. Two patients had exon 10 and 15 mutations, including A366D, H412H, E627D, D641N, and H643D; 5 had N653H. The most frequent mutation of the FGFR3 gene was identified at R248C. None of the inverted papillomas exhibited mutations in TP53. The markedly reduced frequency of LOH, the absence of TP53 mutations, the absence of telomere shortening, and the pattern of FGFR3 mutations in inverted papilloma in contrast to that of urothelial carcinoma suggest that inverted papilloma does not harbor the key genetic abnormalities that predispose to the development of urothelial carcinoma and may indicate that these entities arise through separate and distinct pathogenetic mechanisms.21–23,44 Squamous papilloma is a rare benign neoplasm; it may represent the squamous counterpart of urothelial papilloma.45 It is unrelated to human papillomavirus (HPV) infection, usually occurs in elderly women, and follows a benign clinical course.45 Histologically, it is composed of papillary cores with overlying benign squamous epithelium (Fig. 6-5). These tumors are diploid with undetectable or very limited nuclear p53 accumulation. Some demonstrate immunohistochemical expression of epidermal growth factor receptor (EGFR) protein.46 Papillary urothelial hyperplasia typically occurs in patients with either a prior or concurrent low-grade papillary urothelial neoplasia.47 Most patients are men, with a mean age of 67 years. These lesions are characterized by undulating folds of urothelium that lack cytologic atypia and fibrovascular cores.33,47,48 Cytologically, the cells in papillary hyperplasia lack atypia and maintain nuclear polarity. There may be increased vascularity in the stroma at the base of the papillary folds. Data have suggested that papillary hyperplasia may be a precursor to low-grade papillary neoplasms.33,47,48 Swierczynski and colleagues49 reported 15 patients with papillary urothelial hyperplasia with varying degrees of atypia ranging from dysplasia to flat carcinoma in situ (atypical papillary urothelial hyperplasia). On follow-up examination, most of these patients had developed high-grade urothelial neoplasms. The classification of nonpapillary (flat) intraepithelial lesions and conditions of the urothelium has evolved over the years and was redefined at the International Consultation on the Diagnosis of Noninvasive Urothelial Neoplasms held in Ancona, Italy in 2001 (Table 6-1).50 This classification includes epithelial abnormalities (reactive urothelial atypia and flat urothelial hyperplasia), presumed preneoplastic lesions and conditions (keratinizing squamous and glandular metaplasia, and malignancy-associated cellular changes), and preneoplastic (dysplasia) and neoplastic noninvasive (carcinoma in situ) lesions.50,51 Each of these lesions is defined with strict morphologic criteria to provide more accurate information to urologists in managing patients. Table 6-1 Classification of flat urothelial lesions of the urinary bladder based on the Ancona International Consultation Great advances have been made in the molecular genetic and biomarker characterization of bladder cancer.13,37,40,42,52–71 Malignancy-associated cellular change is a concept introduced in the last decade, encompassing urothelial abnormalities in bladders harboring neoplasia that are not evident by routine light microscopy but are demonstrable by chromatin analysis or genetic studies.50,72–74 The clinical relevance of malignancy-associated cellular changes remains to be established, but these parameters may be important in evaluating the status of residual urothelium after surgical bladder tumor resections.73 Studies have shown that 50% of the histologically normal urothelium adjacent to superficial urothelial carcinoma harbors genetic anomalies on chromosome 9, similar to the anomalies found in the coexisting carcinoma. In addition, nondiploid nuclear DNA histograms occur in 4% to 54% of histologically normal urothelium adjacent to bladder tumors.72 These genetic alterations suggest a neoplastic potential for flat urothelial lesions, regardless of whether cytologic atypia is present. Normal urothelium is a multilayered epithelium comprising basal, intermediate, and superficial cells (Fig. 6-6, A). The number of cell layers (usually less than seven) may vary because of tangential sectioning.51 Urothelial hyperplasia is characterized by markedly thickened mucosa with an increase in the number of cell layers, usually 10 or more (Fig. 6-6, B). However, it is not necessary to count the number of cell layers for the diagnosis (Table 6-2). The cells in urothelial hyperplasia do not show any significant cytologic abnormalities, although slight nuclear enlargement may be focally present. Morphologic evidence of maturation from base to surface is generally evident. Urothelial compression artifact or tangential sectioning of mucosa with pseudopapillary growth (lacking a true vascular core) may resemble flat urothelial hyperplasia. Fig. 6-6 Flat intraepithelial lesions. A, Normal urothelium. B, Urothelial (simple) hyperplasia. C, Reactive atypia. D, Urothelial dysplasia. E, Urothelial carcinoma in situ. Flat urothelial hyperplasia has been observed in association with a variety of conditions, including inflammatory disorders, urolithiasis, papillary urothelial hyperplasia, dysplasia, carcinoma in situ, and low-grade papillary tumors.50 When seen as an isolated phenomenon, no evidence suggests that primary urothelial hyperplasia has a premalignant potential. However, molecular analyses showing chromosome 9q deletions and mutations in the FGFR3 gene in both urothelial hyperplasia and low-grade papillary neoplasia16 suggest that this lesion may be clonally related to the papillary tumors in patients with bladder cancer.75,76 Flat urothelial hyperplasia has been considered by some authors to be the source of papillary neoplasia, usually associated with low-grade tumors.77 Urothelial abnormalities whose architectural and cytologic changes are of lesser degree than those of dysplasia often have been termed atypia.50 The term atypia is, by its very nature, nonspecific. The intraobserver and interobserver variation in recognition and interpretation of “urothelial atypia” is substantial. Nevertheless, the term atypia is still in use at many institutions. Two similar categories of atypia have been recognized, namely reactive atypia and atypia of unknown significance.78 Both are placed among the “benign” urothelial abnormalities.50 Reactive atypia is characterized by mild nuclear abnormalities occurring in acutely or chronically inflamed urothelium. In most cases, the patient has a history of cystitis, instrumentation, infection, stones, or previous therapy.51 The epithelium may or may not be thickened in reactive atypia. The cells are often larger than normal, with more abundant cytoplasm than normal urothelial cells (Fig. 6-6, C). These features occasionally impart a squamoid appearance. Nuclei are uniformly enlarged and vesicular and may have prominent, usually centrally located, nucleoli. Mitotic figures may be frequent but always occur in the lower epithelial layers. Inflammatory cells occupying the lamina propria and infiltrating into the urothelium are invariably present. CD44 and p53 immunohistochemical stains may be particularly useful from a differential diagnosis perspective (see later discussion of urothelial dysplasia). The term atypia of unknown significance was introduced by the International Society of Urological Pathology (ISUP) consensus group to describe lesions in which the pathologist was uncertain whether the changes were reactive or preneoplastic.78 Atypia of unknown significance is characterized by nuclear changes similar to those seen in reactive atypia. However, the degree of nuclear pleomorphism and hyperchromasia is greater than in reactive atypia and dysplasia cannot be definitely ruled out. Inflammation in the lamina propria with urothelial infiltration often is present. However, the cellular changes seem to be disproportionate to the degree of inflammation. Atypia of unknown significance often is seen in patients with a previous diagnosis of urothelial neoplasia. Progression to urothelial carcinoma has not been documented.51 Currently, no evidence exists supporting a premalignant nature of such lesions. The clinical outcome of patients with atypia of unknown significance is identical to that of patients with reactive atypia.51 The utility of creating this diagnostic category has been questioned, and the use of the designation “atypia of unknown significance” is discouraged. Urothelial dysplasia is defined as abnormal urothelium with cytologic and architectural changes that do not meet all the criteria for an unequivocal diagnosis of urothelial carcinoma in situ (Figs. 6-6, D, 6-7, and 6-8).50,51,79,80 The overall appearance is that of the urothelium in low-grade papillary urothelial carcinoma. The cytologic abnormalities in urothelial dysplasia, characterized by cellular crowding, loss of orderly maturation, and loss of cellular polarity are not present in the full thickness of the urothelium (see Figs. 6-6, D, 6-7, and 6-8). Occasionally, an increased number of cell layers may be present. The superficial umbrella cells are usually present. Most cellular abnormalities in dysplasia are restricted to the basal and intermediate cell layers. Individual dysplastic cells show enlarged nuclei and nucleoli with irregular contours and coarsening of the chromatin. Multiple nucleoli and nuclear overlapping may be seen. The cells often show cytoplasmic clearing. Mitotic figures, when present, are generally basally located. The transition from normal to abnormal urothelium is subtle, and nondysplastic urothelial cells are often dispersed among the dysplastic cells. Fig. 6-7 Morphologic continuum from normal urothelium through dysplasia to carcinoma in situ, and invasion. The dysplastic urothelium shows loss of orderly maturation and cellular polarity. The progression from dysplasia to carcinoma in situ is characterized by the increasing nuclear-to-cytoplasmic ratio, nuclear hyperchromasia, and nuclear and nucleolar enlargement. The superficial umbrella cell layer is often absent in urothelial carcinoma in situ, but its loss is not a prerequisite for the diagnosis. Urothelial carcinoma in situ often progresses to invasive cancer. Fig. 6-8 Urothelial dysplasia. Dysplastic urothelium shows variability in nuclear size and shape, increase in nuclear-to-cytoplasmic ratio, and loss of cellular polarity. Cytoplasmic clearing is common. The superficial umbrella cell layer is intact. The degree of cytologic atypia is insufficient for an unequivocal diagnosis of carcinoma in situ. The diagnosis of urothelial dysplasia can be made in cases in which the urothelium demonstrates significant cytologic atypia that cannot be attributed to inflammation or a reparative process and yet lacks the full complement of cytologic abnormalities that characterize carcinoma in situ. Nuclear and architectural features are the primary criteria for distinguishing dysplasia from reactive atypia and urothelial carcinoma in situ. Aberrant CK20 expression in urothelial cells plus overexpression of p53 and Ki67 are indicators of dysplastic change in urothelial mucosa (Table 6-3).80–85 Molecular markers may be helpful in the differential diagnosis. CK20 immunostaining is limited to the superficial cell layers in normal urothelium; in contrast, CK20 immunostaining is usually present in the superficial and intermediate cell layers of dysplastic urothelium.81,86 CD44 may be of particular value in the distinction between urothelial dysplasia and reactive atypia.82,87 Positive CD44 immunostaining is observed only in the basal cells in normal urothelium, and is either absent entirely or present only in scattered cells in urothelial dysplasia, whereas full-thickness positive membranous CD44 staining is typical of reactive urothelium.87 Dysplastic cells show increased p53 expression, whereas p53 nuclear accumulation is predominantly undetectable or only weakly evident in the basal and parabasal cells in reactive urothelium. Alterations of p53 and allelic losses, particularly in chromosome 9, may occur in dysplasia.76 Primary dysplasia occurs in the absence of other urothelial tumors. Its prevalence in the general population is unknown because of the lack of large-scale screening studies. In an autopsy series of 313 patients without gross lesions, urothelial dysplasia was present in 6.8% of males and 5.7% of females.88 Only a few studies provide clinical information on patients with primary dysplasia.51,79,89,90 These patients are predominantly middle-aged men with irritative symptoms with or without hematuria. The lesion has a predilection for posterior wall location.79 Dysplasia is not cystoscopically visible, although occasionally the urothelium may appear raised and irregular or mildly erythematous. It is estimated that de novo (primary) dysplasia progresses to bladder neoplasia in 14% to 19% of patients.51,79,89,90 Using modern criteria for urothelial dysplasia, Cheng and associates79 found a 19% progression rate in 36 patients with isolated urothelial dysplasia during a mean follow-up of 8.2 years. A similar progression rate (15%) was found in a different cohort of patients.51 Secondary dysplasia is seen in patients with a history of bladder neoplasia. The incidence of dysplasia in patients with established bladder neoplasia varies from 22% to 86% and approaches 100% in patients with invasive carcinoma.50,91–96 As many as 24% of random biopsies from patients with stage Ta and T1 carcinoma show epithelial abnormalities that include dysplasia and carcinoma in situ.97 The presence of urothelial dysplasia indicates urothelial instability and is a harbinger of recurrence and progression.72,96,98–104 The recurrence rate was 73% of patients with superficial neoplasia and concomitant dysplasia in contrast to 43% without coexisting dysplasia.97 Of the 30% of patients with superficial urothelial carcinoma who developed muscle-invasive cancer within 5 years after the initial diagnosis, most had dysplasia or carcinoma in situ adjacent to the primary tumor.98 Of the patients with dysplasia elsewhere in the bladder mucosa, 36% eventually developed muscle-invasive tumors, whereas only 7% of patients with normal urothelium in adjacent biopsies subsequently developed muscle-invasive cancer. Urothelial carcinoma in situ is a flat noninvasive lesion in which the urothelium is entirely composed of cytologically malignant cells. Melamed and colleagues105 first described the natural history of urothelial carcinoma in situ and found that 9 of 25 patients (36%) developed invasive carcinoma within 5 years after the initial diagnosis. In the study by Cheng and associates of 138 patients with urothelial carcinoma in situ in the absence of invasive cancer, the patient’s age at diagnosis ranged from 32 to 90 years (mean, 66 years).106 The male-to-female ratio in patients with carcinoma in situ is approximately 7 : 1. Clinical presentations include gross and microscopic hematuria, irritative symptoms (dysuria, pain, frequency), nocturia, and sterile pyuria. Approximately 25% of patients are asymptomatic. Carcinoma in situ usually is multifocal, with a predilection for the trigone, lateral wall, and dome of the bladder. Cystoscopically, it may appear as erythematous velvety or granular patches, although it also may be visually undetectable. Erythematous changes are often apparent at gross examination (Fig. 6-9). De novo or isolated carcinoma in situ (often referred to as primary carcinoma in situ) accounts for 1% to 3% of bladder neoplasms.12,50,106–111 The clinical manifestation often closely mimics that of interstitial cystitis. Urothelial carcinoma in situ is very often multifocal. Mapping studies of cystectomy specimens show extensive carcinoma in situ, with involvement of the prostatic urethra and of the ureter in as many as 67% and 57% of patients, respectively.112–118 Urothelial carcinoma in situ has a high likelihood of progressing to invasive carcinoma if left untreated.106 The mean interval between a diagnosis of carcinoma in situ and the detection of cancer progression is 5 years. The actuarial progression-free survival, cancer-specific survival, and all-cause survival rates are 63%, 79%, and 55%, respectively, at 10 years and 59%, 74%, and 40%, respectively, at 15 years (Fig. 6-10).106 Factors predictive of progression include multifocality, coexistent bladder neoplasia, DNA aneuploidy, CCND3 gene amplification, prostatic urethral involvement, and recurrence after treatment.† Fig. 6-10 Kaplan-Meier survival curves for 138 patients with primary urothelial carcinoma in situ of the bladder. No patients had invasive urothelial carcinoma at the time of diagnosis. The numbers in parentheses represent the number of patients under observation at 5, 10, and 15 years. Progression was defined as development of invasive carcinoma, distant metastasis, or death from bladder cancer. (From Cheng L, Cheville JC, Neumann RM, et al. Survival of patients with carcinoma in situ of the urinary bladder. Cancer 1999;85:2469-2474, with permission.) Urothelial carcinoma in situ is often associated with invasive carcinoma elsewhere in the bladder (referred to as secondary carcinoma in situ).122 The frequency of carcinoma in situ increases with the grade and stage of the associated urothelial neoplasm. Patients with coexisting invasive urothelial carcinoma have higher risk for cancer progression and cancer-specific death than patients with primary carcinoma in situ.50 Urothelial carcinoma in situ is characterized by flat disordered proliferation of urothelial cells with marked cytologic abnormalities (Fig. 6-11). The morphologic diagnosis of carcinoma in situ requires severe cytologic atypia (anaplasia).12,50,106 Full-thickness change, which may range from one cell layer to the thickness of hyperplasia (greater than seven cells) is not required. Superficial (umbrella) cells may be present. Marked disorganization of cells is characteristic, with loss of cellular polarity and decreased cellular cohesiveness. The tumor cells tend to be large and pleomorphic, with moderate-to-abundant cytoplasm. Nevertheless, the cells of carcinoma in situ are sometimes small, with a high nuclear-to-cytoplasmic ratio. The chromatin tends to be coarse and clumped. Nucleoli may be multiple and are large and prominent in at least some of the cells. Mitotic figures, which are often atypical, are seen in the uppermost layers of the urothelium. The adjacent mucosa often contains lesser degrees of cytologic abnormality. Tissue edema, vascular ectasia, and proliferation of small capillaries are frequently observed in the lamina propria (see Fig. 6-11). Fig. 6-11 Urothelial carcinoma in situ displaying disordered proliferation of malignant urothelial cells that demonstrate a high nuclear-to-cytoplasmic ratio, nuclear pleomorphism, irregular nuclear contours, coarsely granular chromatin, and prominent nucleoli. A to C, Loss of cellular polarity and cell cohesion are seen in urothelial carcinoma in situ. Vascular proliferation is often prominent in the underlying stroma. Full-thickness involvement is not required for the diagnosis of urothelial carcinoma in situ. The superficial umbrella cell layer may still be present. D, Aberrant expression of cytokeratin 20 is observed. An immunohistochemical panel, consisting of CK20, CD44, p53, and Ki67 may be helpful in differential diagnosis.50,81,82,123–127 Carcinoma in situ shows intense CK20 and p53 positivity in the majority of malignant cells. Increased Ki67 labeling is noted in carcinoma in situ but also can be seen in reactive atypia of the urothelium, thus limiting its usefulness in practice. The neoplastic cells are uniformly negative for CD44 immunostaining.123 In contrast, CK20 staining shows patchy cytoplasmic immunoreactivity only in the superficial umbrella cell layer and CD44 stains only the basal cells in the adjacent normal urothelium. Morphologic variants and growth patterns of carcinoma in situ have been recognized over the years (Table 6-4). Although it is not necessary to mention these specific growth patterns or morphologic variants in the surgical pathology report, awareness of the histologic diversity of carcinoma in situ may aid in the diagnosis of this therapeutically and biologically important lesion.50,128 These lesions may be associated with microinvasion, sometimes clinically unsuspected and histologically subtle. Large cell carcinoma in situ constitutes the most common morphologic form of this entity. Cytologic findings include nuclear pleomorphism, variably abundant cytoplasm, and anaplastic nuclear features (Fig. 6-12, A). In rare cases, large cell carcinoma in situ may have minor nuclear pleomorphism but still exhibits architectural disarray. Rare cases may focally exhibit high pleomorphism with bizarre giant cells. Fig. 6-12 Histologic variants of urothelial carcinoma in situ. A, Large cell carcinoma in situ. B, Small cell carcinoma in situ. Small cell carcinoma is present in the lamina propria. C, “Clinging” carcinoma in situ. D, Denuding carcinoma in situ. The urothelium is partially denuded; residual carcinoma in situ cells are present in the remainder of the urothelium. The small cell pattern refers to the size of the cells and may or may not coexist with small cell carcinoma (see Fig. 6-12, B); it is unrelated to neuroendocrine differentiation of carcinoma in situ. In such cases, the pleomorphism is usually minimal, the cytoplasm is scant, and the nuclei are enlarged and hyperchromatic, with coarse, unevenly distributed chromatin. The scattered prominent nucleoli are distorted and angulated. Recognition of the small cell pattern of carcinoma in situ is important to avoid misdiagnosis of basal cell hyperplasia, which has been observed in patients treated with bacille Calmette-Guérin (BCG). These show a small cell pattern but lack nuclear atypia or loss of polarity. In some cases, the neoplastic urothelial cells are strikingly dyscohesive and undergo extensive exfoliation, with the result that biopsies may show only a few residual carcinoma cells on the surface (“clinging carcinoma in situ”) or no recognizable epithelial cells on the surface, a condition referred to as denuding cystitis (see Fig. 6-12, C and D).129 In the clinging pattern of carcinoma in situ, a patchy, usually single layer of atypical cells is seen. In mucosal biopsies entirely lacking surface epithelium, carcinoma in situ may be present only in von Brunn nests. A careful search for carcinoma in situ in deeper sections or in other submitted biopsy fragments is important, and a recommendation for evaluation of urine cytology for carcinoma cells is warranted. Another pattern of carcinoma in situ, also referred to as cancerization of the urothelium, shows either pagetoid spread (clusters or isolated single cells) or undermining or overriding of the normal urothelium (lepidic growth) (Fig. 6-13). Carcinoma in situ exhibiting pagetoid growth is characterized by large single cells or small clusters of cells within otherwise normal urothelium of the ureter, urethra, or prostatic ducts or in areas of squamous metaplasia. Individual cells showing pagetoid spread have enlarged nuclei with coarse chromatin; frequently, the cytoplasm is clear. Fig. 6-13 Pagetoid and lepidic urothelial carcinoma in situ. A to C, Pagetoid spread of urothelial carcinoma in situ. Clusters and isolated single neoplastic cells (B and C) are present in the urothelium. D, Urothelial carcinoma in situ also may display an undermining or lepidic growth pattern. Pagetoid growth patterns can be found in up to 15% of carcinoma in situ cases.50 Most patients are male and their ages range from 31 to 78 years (mean, 64 years). Pagetoid carcinoma in situ is usually a focal lesion and is easily overlooked. It occurs in a clinical and histologic setting of conventional carcinoma in situ with coexisting invasive urothelial carcinoma; these patients essentially have the same progression and survival rates as patients without pagetoid changes. In cases with extensive urothelial denudation, pagetoid carcinoma in situ may be focally present in adjacent otherwise normal-appearing urothelium, thus alerting the surgical pathologist to search for additional carcinoma in situ elsewhere in the bladder. Primary extramammary Paget disease of the external genitalia and anal canal may extend to the bladder, and, conversely, some cases of pagetoid carcinoma in situ of the bladder may extend to the urethra, ureter, and external genitalia; therefore differentiating between these two entities represents an important diagnostic and therapeutic challenge. A panel of immunostains including CK7, CK20, and thrombomodulin may assist in differentiating pagetoid urothelial carcinoma in situ from extramammary Paget disease, which is known to be CK7 positive and CK20 negative.50 Rare cases of carcinoma in situ may exhibit squamous differentiation characterized by intercellular bridges. Carcinoma in situ with squamous features is most often observed in association with urothelial carcinoma showing extensive squamous differentiation elsewhere in the bladder (Fig. 6-14, A). A much less frequently encountered pattern is carcinoma in situ with morphologic and immunohistochemical evidence of glandular differentiation (see Fig. 6-14, B). Some authors refer to this as adenocarcinoma in situ; such lesions may show papillary, cribriform, or flat morphology. Carcinoma in situ involving von Brunn nests (see Fig. 6-14, C), cystitis glandularis, or cystitis cystica may be difficult to distinguish from adenocarcinoma in situ in the absence of concurrent invasive adenocarcinoma. A recent report based on 25 patients with urothelial carcinoma in situ with glandular differentiation showed high Ki67 index and p53 accumulation, high nuclear and cytoplasmic p16 expression, and diffuse primitive neuroectodermal tumor expression, a phenotype that also characterized concurrent conventional carcinoma in situ.130 MUC5A, MUC2, CK20, and HER2 were positive in all 25 patients with urothelial carcinoma in situ with glandular differentiation, and CDX2 was present in 19 patients; MUC1, CK7, or CK34βE12 was focally present in 21, 19, and 18 patients, respectively. The authors concluded that urothelial carcinoma in situ with glandular differentiation is a variant of carcinoma in situ that follows the natural history of conventional urothelial carcinoma in situ. The immunophenotype suggests urothelial origin with the expression of MUC5A and CDX2 as signature for glandular differentiation.130 Fig. 6-14 Urothelial carcinoma in situ may show squamous (A) or glandular differentiation (B). Urothelial carcinoma in situ involving von Brunn nest (C) may be misdiagnosed as adenocarcinoma in situ. D, Carcinoma in situ is often associated with invasive urothelial carcinoma (gross appearance). E, Carcinoma in situ with microinvasion is characterized by individual single cells in the stroma. The adjacent von Brunn nests are also involved by carcinoma in situ. Carcinoma in situ with microinvasion was initially defined by Farrow and colleagues as invasion into the lamina propria to a depth of 5 mm or less from the basement membrane (see Fig. 6-14, D and E).131 An international consensus conference suggested that cases with more than 20 cells measured from the stromal–epithelial interface should be classified as fully invasive.50 Microinvasion appears as direct extension in cords (tentacular), single cells, or single cells and clusters of cells (see Fig. 6-14, E). The clusters of cells may have retraction artifact that mimics vascular invasion. Stromal response may be present but in most cases is absent. In cases with a prominent stromal inflammatory response, the invasive neoplastic cells may be interspersed among lymphocytes, making them inconspicuous. In these circumstances, immunohistochemical staining with antibodies against cytokeratins (e.g., AE1/AE3) exposes the invading cells.132 Antineoplastic agents used in the bladder or systemically, such as thiotepa (triethylenethiophosphoramide), mitomycin C, cyclophosphamide, BCG, and radiation therapy, produce urothelial changes that can mimic cancer histologically. Pathologists must be aware of the diagnostic pitfalls and exercise caution when evaluating urothelial atypia after treatment with chemotherapy or irradiation.50,133–136 In most cases, knowledge of the prior treatment is crucial to correctly diagnosing the epithelial and stromal changes present (Fig. 6-15). If the distinction between treatment-induced atypia and dysplasia or carcinoma in situ is unclear, a conservative approach with repeat cystoscopy and biopsy is indicated, preferably after the inflammation has subsided. Fig. 6-15 Cyclophosphamide (Cytoxan)-induced changes include urothelial atypia and hemorrhagic cystitis. Cyclophosphamide therapy may induce stromal fibrosis, vascular intimal thickening, mural fibrin deposition in vessels, and vascular ectasia.134 It also induces epithelial necrosis followed by rapid atypical regeneration. The metabolic effects of cyclophosphamide, including arrest of cell and nuclear division, produce binucleated and multinucleated cells, often with large bizarre nuclei resembling radiation injury changes that can be mistaken for malignancy. Cyclophosphamide also may induce reactivation of polyomavirus infection causing marked nuclear atypia in the surface urothelium. In rare cases, polyomavirus infection (with BK virus) mimics flat intraepithelial lesions in immunocompromised patients. In BCG-treated bladders, it is important to keep in mind that residual carcinoma in situ might be present only in von Brunn nests. Loss of intercellular cohesion in carcinoma in situ may result in the so-called denuding cystitis or in residual neoplastic cells loosely attached to the surface (“clinging” pattern). Also of importance are the reactive changes associated with BCG therapy, which include both acute and chronic inflammation. A pattern of reactive epithelial atypia and granulomatous reaction deep in the bladder wall may be seen.134,135 Mitomycin C and thiotepa, when used as topical chemotherapeutic agents in the bladder, produce identical histologic changes. These include exfoliation, epithelial denudation, multinucleation, cytoplasmic vacuolization, and the appearance of bizarre, nonmalignant nuclei in the superficial layer of the urothelium. A marked necroinflammatory process follows administration of topical mitomycin C. A histiocytic response extends deep into the bladder wall. Mitomycin C also may initiate eosinophilic cystitis, a useful clue for the surgical pathologist when evaluating small bladder biopsies in this setting. These agents are not metabolic inhibitors of DNA replication and thus do not produce full-thickness urothelial atypia, as is seen after cyclophosphamide therapy. Patients receiving ketamine may present reactive urothelial changes that can mimic urothelial carcinoma in situ.137 Radiation therapy produces a variety of bladder lesions associated with a progression of pathologic findings. The earliest change, usually seen after 3 to 6 weeks, consists of acute cystitis with desquamation of urothelial cells and hyperemia with edema of the lamina propria. The urothelium shows varying degrees of atypia, including cytoplasmic and nuclear vacuolization, karyorrhexis, stromal hyalinization, thrombosis of blood vessels, and mesenchymal cell atypia similar to that seen in giant cell cystitis.134 Enlarged nuclei may have large nucleoli, but degenerative nuclear features usually are present. Surface ulceration with fibrin deposition, or a reactive, tumor-like epithelial proliferation associated with fibrosis of the lamina propria or muscularis propria, arteriolar mural thickening and hyalinization, and atypical and sometimes multinucleated stromal cells are features seen in late cases of radiation cystitis, usually becoming evident months or years after radiation therapy. An important long-term effect of radiotherapy is the development of de novo radiation-induced bladder cancer, which usually is a urothelial carcinoma but occasionally is a squamous cell neoplasm. Rare examples of sarcomatoid carcinoma (or carcinosarcoma) and sarcoma of the urinary bladder have been reported.50,134,135,138–140 Several inflammatory conditions of the urinary bladder, some of them related to treatment, may cause urothelial atypia leading to overdiagnosis of dysplasia and carcinoma in situ. In addition, the damaged mucosa may become ulcerated, with adjacent atypical regenerating urothelium showing pseudoepitheliomatous hyperplasia.50,135,141,142 This change is more common after ulceration related to radiation therapy. Bladder cancer is the seventh most common cancer worldwide, accounting for approximately 336,000 new cases each year.2-5,120,143–146 Significant variations are found in incidence, morbidity, recurrence, progression, and mortality rates of bladder cancer in different countries and ethnicity groups.147–154 African-American men have a much lower incidence of bladder cancer, but their mortality rates are similar to those of whites.120,146,155–157 Bladder cancer occurs two to five times more frequently in men than women. This has been attributed to different smoking and occupational exposure between men and women.120,146,155,156 Multiple risk factors have been linked to bladder cancer. Exogenous factors, such as tobacco smoking, occupational risk, and lifestyle exposure to carcinogens all play important roles. Smokers have two to four times the risk for urothelial cancer than the general population, and heavy smokers have five times the risk.156 Nevertheless, the specific urothelial carcinogens associated with smoking are still unknown. It is estimated that 20 years of smoking is needed for development of bladder cancer, and the probability of this event is directly correlated with the lifetime number of cigarettes consumed. The relative risk for active smokers developing bladder cancer in contrast to people who never smoked is 3 : 1, and for previous smokers it is 1.9 : 1. Although the exact mechanism by which tobacco causes bladder cancer is not known, many known carcinogens in cigarette smoke, such as acrolein, 4-amino-biphenyl, arylamine, and oxygen free radicals, have been implicated. Furthermore, increased duration, intensity of tobacco consumption, and degree of inhalation significantly contribute to cancer development. The beneficial effects of smoking cessation, on the other hand, include an almost immediate decline in risk for bladder cancer. Continued smokers have a worse recurrence-free survival than those who quit at the time of diagnosis.120,158 Ex-smokers and current smokers also have a significantly shorter recurrence-free survival after treatment for bladder cancer.159 Occupational exposure to aniline dyes and aromatic amines such as 2-naphthylamine and benzidine is the second most prevalent risk factor for bladder cancer. Benzidine, the most carcinogenic aromatic amine, is used in dye production and as a hardener in the rubber industry.158 The degree of carcinogenesis resulting from occupational exposure varies with the degree of industrialization, but in heavily industrialized nations, occupational exposure may account for up to one fourth of all urothelial cancers. The latency period between exposure and tumor development is usually prolonged.120 Occupational bladder cancer also has been observed in gas workers, painters, and hairdressers. Nutrition also may play a role. Vitamin A supplementation apparently reduces the risk for bladder cancer, whereas fried food and fat ingestion cause a risk increase. A high fluid intake reduced the risk for bladder cancer in one study, but this remains controversial. Epidemiologic studies in Taiwan and Chile have shown an increased risk for urothelial cancer in people whose drinking water has a high content of arsenic. Other water contaminants with putative toxic effects on urothelium are actively being investigated.120 Additional factors implicated in the development and progression of bladder cancer include analgesic use, urinary tract infections (bacterial, parasitic, fungal, or viral), urinary lithiasis, pelvic radiation, and chemotherapeutic agents such as cyclophosphamide.160 Although caffeine ingestion has been implicated as a risk factor for bladder cancer, risk estimates for this association decrease after controlling for concomitant tobacco use. Similarly, saccharin-containing artificial sweeteners have induced bladder neoplasia in rats, but human epidemiologic studies have failed to establish this relationship in humans. A relationship exists between the parasite Bilharzia (schistosomiasis) and squamous cell cancer in the bladder, more frequently seen in the Middle East, where the water-borne flatworms are endemic.120 The initial evaluation and management for patients with suspected bladder cancer involves cystoscopic evaluation of the bladder and prostatic urethra for mucosal lesions. Small lesions and flat lesions worrisome for carcinoma in situ can be sampled with cold-cup biopsy forceps, and larger suspicious lesions are resected transurethrally as completely as possible. Transurethral resections and biopsies should include muscularis propria if possible.161 Tumor recurrence is a significant risk factor for cancer progression.162–165 New guidelines of the International Consultation on Urological Diseases were recently published.166–171 Development of multifocal tumors in the same patient, either synchronous or metachronous, is a common characteristic of urothelial malignancy (Fig. 6-16).‡ Multiple coexisting tumors often have arisen before clinical symptoms are apparent. The separate tumors may or may not share a similar histology. Two theories have been proposed to explain the frequency of urothelial tumor multifocality. One theory—the monoclonal theory—suggests that the multiple tumors arise from a single transformed cell that proliferates and spreads throughout the urothelium by either intraluminal implantation or intraepithelial migration. The second theory—the field effect theory—explains tumor multifocality as a development secondary to field cancerization effect. Chemical carcinogens cause independent transforming genetic alterations at different sites in the urothelial lining, leading to multiple genetically unrelated tumors. Fig. 6-16 Gross appearance of multifocal urothelial carcinoma. The papillary architecture of these tumors is apparent. The issue of monoclonal versus oligoclonal origin of multifocal urothelial carcinomas is clinically important for understanding patterns of early tumor development when planning treatment and surgical strategies.§ The cause of multifocality also influences test design for genetic detection of recurrent or residual tumor cells in posttreatment urine samples. Currently, no consensus exists concerning which theory is most important in the development of multifocal urothelial carcinoma. Many studies have suggested a monoclonal origin for multifocal urothelial carcinoma,178–189 but other studies have shown an independent origin for some multicentric urothelial tumors using similar methods. Field cancerization, which is an important cause of multicentric squamous cell carcinomas of the head and neck, postulates that multifocal urothelial carcinomas arise in the same way.61 In the field cancerization process, simultaneous or sequential tumors result from numerous independent mutational events at different sites in the urothelial tract. These independent transformations are a consequence of external cancer-causing influences. In support of the field effect theory is the frequent finding of genetic instability in normal-appearing bladder mucosa in patients with bladder cancer in the adjacent urothelium.43,195 Premalignant changes, such as dysplasia or carcinoma in situ, often are found in urothelial mucosa away from an invasive bladder cancer. Many genetic comparisons and mapping of atypia in cystectomy specimens have emphasized the role of oligoclonality and field cancerization in the development of multifocal urothelial tumors, especially in early-stage disease. The monoclonal and oligoclonal theories to explain urothelial tumor multifocality are not mutually exclusive, and various theories have been proposed to combine the two mechanisms. It has been suggested that oligoclonality is more common in early lesions, with progression to higher stages leading to the overgrowth of one clone and pseudomonoclonality.188,196 Thus early or preneoplastic lesions may arise independently with a specific clone undergoing malignant transformation, which subsequently spreads through the urothelium by either an intraluminal or intraepithelial dissemination. Whereas tumor multifocality seems to be an oligoclonal phenomenon in the majority of cases, undeniable support exists for the monoclonal hypothesis in some cases.40 Histologic grading is one of the most important prognostic factors in bladder cancer.2,197–199 The first widely accepted grading system for papillary urothelial neoplasms was the WHO (1973) classification system, which divided urothelial tumors into four categories: papilloma, grade 1 carcinoma, grade 2 carcinoma, and grade 3 carcinoma.8 Histologic grading is based on the degree of cellular anaplasia, with grade 1 tumors having the least degree of anaplasia compatible with a diagnosis of malignancy, grade 3 tumors the most severe degree of anaplasia, and grade 2 tumors an intermediate degree of cellular anaplasia (Fig. 6-17). Anaplasia is further defined by the authors of the 1973 WHO classification as increased cellularity, nuclear crowding, disturbed cellular polarity, failure of differentiation from the base to the surface, nuclear polymorphism, irregular cell size, variations in nuclear shape and chromatin pattern, displaced or abnormal mitotic figures, and giant cells.8 Fig. 6-17 Gross and microscopic appearance of noninvasive urothelial carcinoma. Grading using the 1973 World Health Organization classification scheme is recommended. A and B, Grade 1 urothelial carcinoma. C and D, Grade 2 urothelial carcinoma. E and F, Grade 3 urothelial carcinoma. The 1973 WHO histologic grading of bladder cancer is one of most successful grading systems among all organ sites and has been validated since its introduction four decades ago.199–202 The 1973 WHO grading system has been accepted by pathologists, urologists, oncologists, and cancer registrars in the United States and elsewhere. An enormous amount of data has been accumulated using this system in studies of the morphologic properties, clinical behavior, treatment, and follow-up of urothelial tumors. Because of its relative simplicity and its well-documented powerful predictive value, it has been well accepted by urologists and used globally in making clinical decisions for management of patients with urothelial cancer for several decades. Grade 1 papillary carcinoma consists of an orderly arrangement of normal urothelial cells lining delicate papillae with minimal architectural abnormality and minimal nuclear atypia. Nuclear grooves are usually present. Some complexity and fusion of the papillae are seen, but this usually is not prominent. The urothelium is often thickened to more than seven cell layers. The urothelium displays normal maturation and cohesiveness, with an intact superficial cell layer. The nuclei tend to be uniform in shape and spacing, although some enlargement and elongation may be seen. The chromatin texture is finely granular, without significant nucleolar enlargement. Mitotic figures are rare or absent and basally located. Grade 1 tumor should be distinguished from urothelial papilloma, which is a benign lesion (Table 6-5). Table 6-5 Diagnostic features of urothelial papilloma and grade 1 (low grade) noninvasive papillary urothelial carcinoma Modified from Cheng L, MacLennan GT, Lopez-Beltran A. Histologic grading of urothelial carcinoma: a reappraisal. Hum Pathol 2012;43:2097-2108. Grade 1 carcinoma appears to have a predilection for the ureteric orifices. In one study, 69% of grade 1 urothelial carcinomas were centered near a ureteric orifice but the remainder was seen in all other portions of the bladder. Patients with grade 1 carcinoma are at increased risk for local recurrence, progression, and dying of bladder cancer. Significant levels of morbidity and mortality are associated with grade 1 urothelial carcinoma of the bladder if patients are followed for a sufficient interval.203–216 With 20 years of follow-up, Holmang and colleagues205 found that 14% of patients with noninvasive grade 1 urothelial carcinoma (pTa G1) died of bladder cancer. In a recent review of 152 patients with stage Ta grade 1 urothelial carcinoma, Leblanc and associates found that 83 patients (55%) had tumor recurrence, including 37% with cancer progression.207 Patients who remained tumor-free for 1 year still had a 43% chance of late recurrence. In the study by Greene and colleagues of 100 patients with grade 1 cancer, 10 patients (10%) died of bladder cancer after more than 15 years; of 73 patients who had recurrences, 22% of recurrences were of higher grade than the original tumor.217 The mean interval from diagnosis to the development of invasive cancer was 8 years. Jordan and associates215 studied 91 patients with grade 1 papillary urothelial tumors and found that 40% of them had recurrence. Of patients with recurrences, 20% developed high-grade (grade 3) cancer, and 4 patients (4%) died of bladder cancer.215 Long-term follow-up is recommended for patients with grade 1 papillary urothelial carcinoma. The prognosis for patients with grade 2 urothelial carcinoma is significantly worse than for those with lower grade papillary cancer. Recurrence risk for patients with noninvasive grade 2 cancer is 45% to 67%.11,12,218 Invasion occurs in up to 20%, and cancer-specific death is expected in 13% to 20% after surgical treatment.11,12,218 Patients with grade 2 cancer and lamina propria invasion are at even greater risk, with recurrences in 67% to 80% of patients, the development of muscle-invasive cancer in 21% to 49%, and cancer-specific death in 17% to 51% of those treated surgically.11,12,218 Some authors consider both nuclear pleomorphism and mitotic count as criteria for subdividing grade 2 urothelial cancer (grades 2A and 2B), and they have been successful in identifying groups of cancers with different outcomes.199,219–222 However, subclassification of grade 2 urothelial carcinoma is not recommended because of significant interobserver variability. Grade 3 carcinoma displays the most extreme nuclear abnormality of any papillary urothelial cancer, similar to changes observed in urothelial carcinoma in situ. The obvious urothelial disorder and loss of polarity is present at scanning magnification. The superficial cell layer is partially or completely absent with grade 3 carcinoma, accompanied by prominent cellular dyscohesion. Obvious loss of normal architecture and cell polarity, and frequent atypical mitotic figures are present. Cellular anaplasia, characteristic of grade 3 carcinoma, is defined as increased cellularity, nuclear crowding, random cellular polarity, absence of normal mucosal differentiation, nuclear pleomorphism, irregularity in cell size, variation in nuclear shape, capricious chromatin pattern, increased frequency of mitotic figures, and occasional neoplastic giant cells.8 Recurrence risk for patients with noninvasive grade 3 cancer is 65% to 85%, with invasion occurring in 20% to 52% and cancer-specific death in up to 35% after surgical treatment.11,223 Of surgically treated patients with grade 3 cancer and lamina propria invasion, 46% to 71% develop recurrences, 24% to 48% develop muscle-invasive cancer, and 25% to 71% suffer cancer-specific death, emphasizing a need for aggressive treatment in these patients.12,218,224 The first widely accepted grading system for papillary urothelial neoplasms was the WHO 1973 classification system.8 In 1998, a revised system of classifying noninvasive papillary urothelial neoplasms of the urinary bladder was proposed.225 This system was subsequently formally adopted by the WHO. In 2004, a classification system for noninvasive papillary urothelial neoplasms, identical to the 1998 WHO/ISUP classification system, was adopted in Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs, one of a series of WHO “Blue Books” for the classification of tumors.5 This new system separates noninvasive papillary urothelial neoplasms into four categories, designated papilloma, papillary urothelial neoplasm of low malignant potential (PUNLMP), low-grade carcinoma, and high-grade carcinoma. The recommendations in this book reflect the views of a working group of urologic pathologists assembled at an Editorial and Consensus Conference held in Lyon, France, in December, 2002, and their subsequently reported findings and recommendations were stated to be a work in progress.5 A PUNLMP is a low-grade urothelial tumor with a papillary architecture and a purported low incidence of recurrence and progression.42,200-202,224,226–234 This lesion is histologically defined by the WHO 2004 classification system as a papillary urothelial tumor that resembles the exophytic urothelial papilloma but with increased cellular proliferation exceeding the thickness of normal urothelium. All such tumors would have been considered grade 1 urothelial carcinomas by the 1973 WHO grading system (Fig. 6-18, I, A). Cytologic atypia is minimal or absent, and architectural abnormalities are minimal, with preserved polarity. Mitotic figures are infrequent and usually limited to the basal layer. Clinically, these tumors show a male predominance (3 : 1) and occur at a mean age of 65 years.235
Neoplasms of the urinary bladder
Benign urothelial (transitional cell) neoplasms
Urothelial papilloma and diffuse papillomatosis
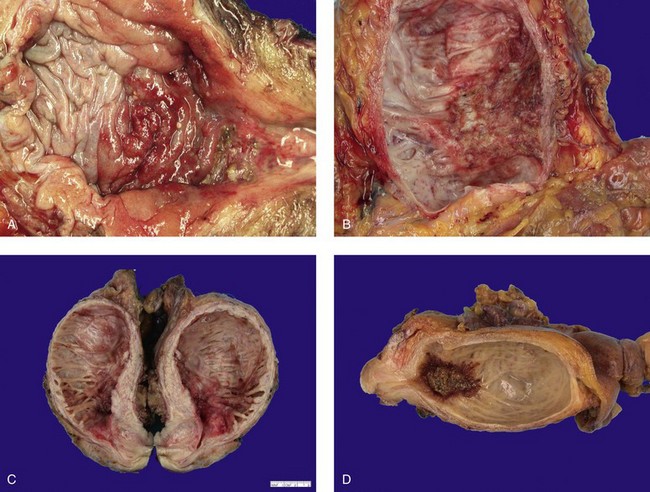
Inverted papilloma
Squamous papilloma
Papillary urothelial hyperplasia
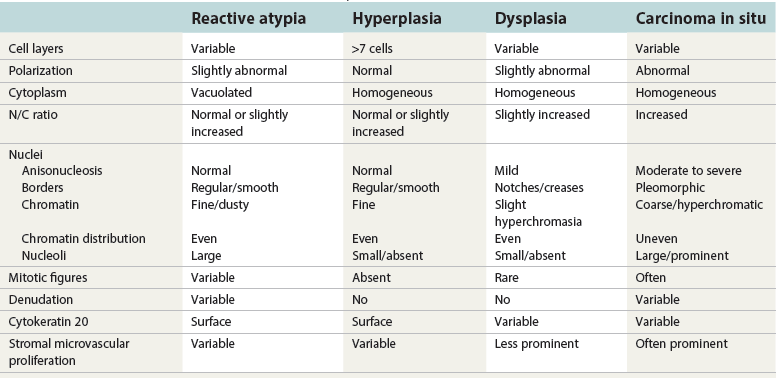
Flat intraepithelial lesions

Flat urothelial hyperplasia (simple hyperplasia)
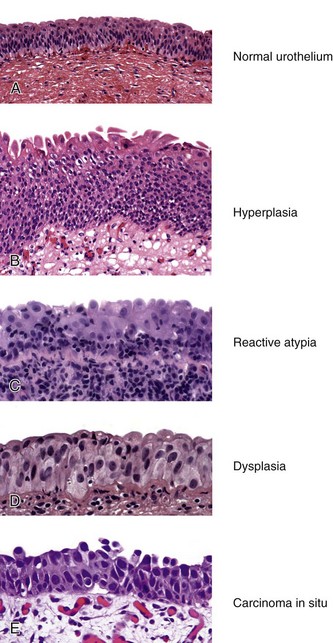
Urothelial reactive atypia
Reactive atypia
Atypia of unknown significance
Urothelial dysplasia (low-grade intraurothelial neoplasia)
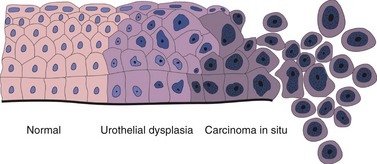
Primary dysplasia
Secondary dysplasia
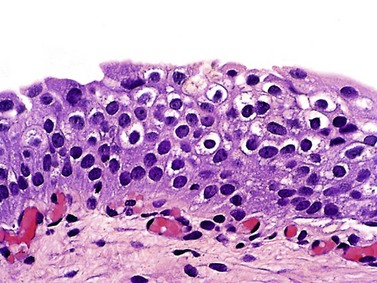
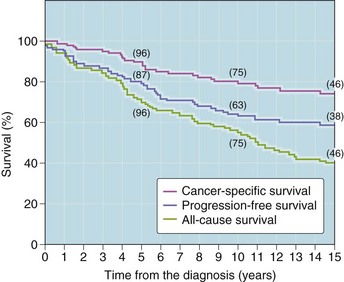
Urothelial carcinoma in situ (high-grade intraurothelial neoplasia)
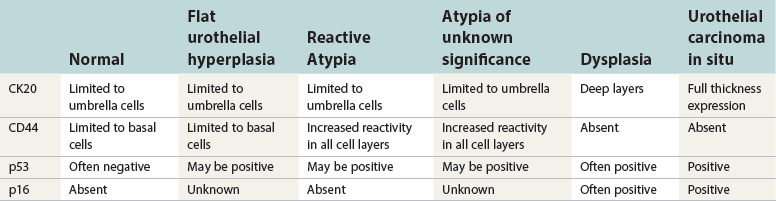
Microscopic pathology
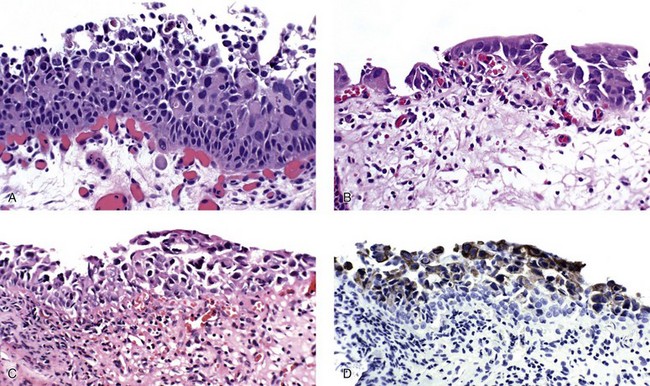
Histologic variants
Large cell carcinoma in situ
Small cell carcinoma in situ
Denuding and “clinging pattern” carcinoma in situ
Pagetoid and undermining (lepidic) carcinoma in situ
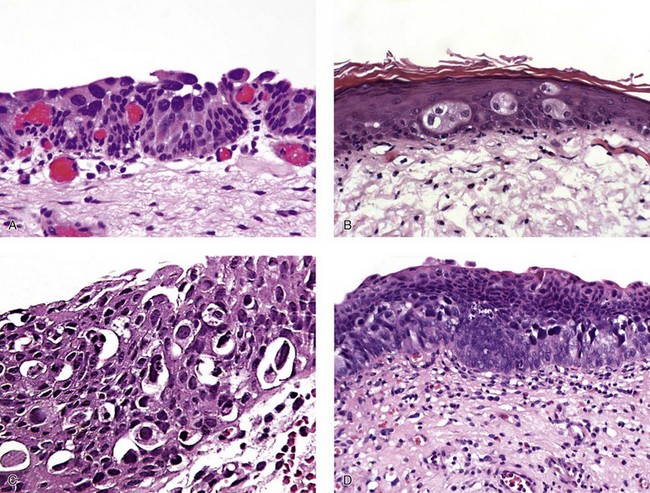
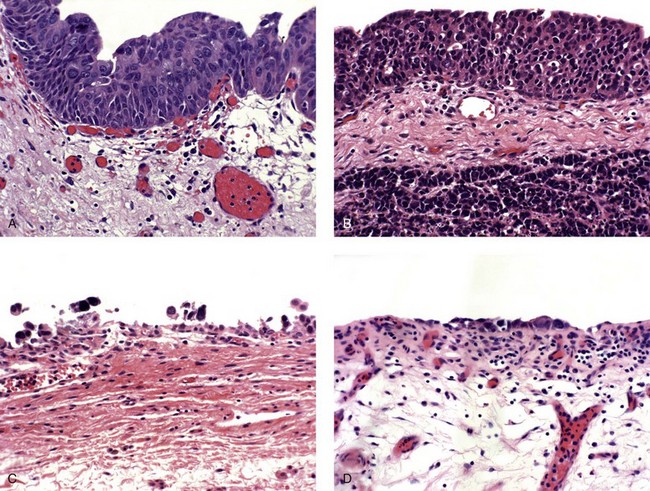
Carcinoma in situ with squamous or glandular differentiation
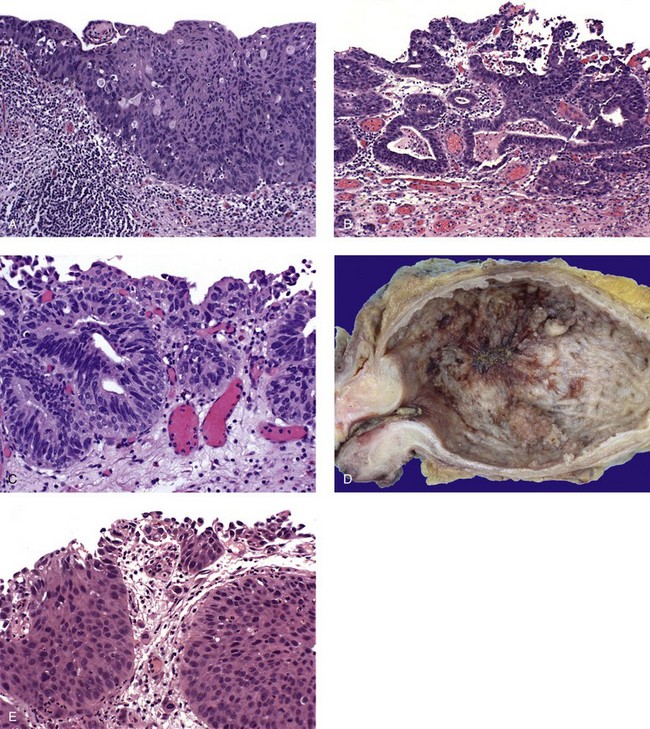
Carcinoma in situ with microinvasion
Therapy-induced changes in the urothelium and mimics of urothelial flat neoplasia
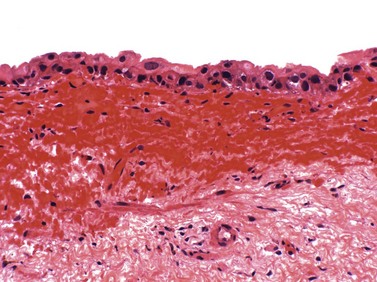
Urothelial (transitional cell) carcinoma
General features
Epidemiology and risk factors
Signs and symptoms
Field cancerization and tumor multicentricity
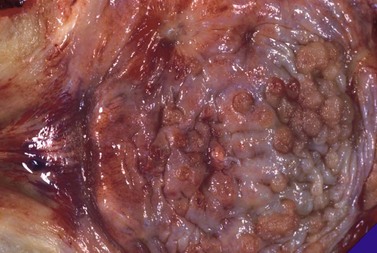
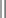 A recent study suggests that both field-cancerization and monoclonal tumor spread may coexist in the same patient.40 Molecular evidence supporting an oligoclonal origin for multifocal urothelial carcinomas in the majority of cases was found, consistent with the field cancerization theory for multicentric urothelial carcinogenesis. This finding is clinically important because an understanding of early tumor development and spread must be considered in the development of appropriate treatment and surgical strategies and when molecular diagnostic techniques are used in the detection of recurrent or residual disease.
A recent study suggests that both field-cancerization and monoclonal tumor spread may coexist in the same patient.40 Molecular evidence supporting an oligoclonal origin for multifocal urothelial carcinomas in the majority of cases was found, consistent with the field cancerization theory for multicentric urothelial carcinogenesis. This finding is clinically important because an understanding of early tumor development and spread must be considered in the development of appropriate treatment and surgical strategies and when molecular diagnostic techniques are used in the detection of recurrent or residual disease.
Histologic grading
Histologic grading according to the 1973 World Health Organization classification
Grade 1 urothelial carcinoma
Urothelial papilloma
Grade 1 (low grade) urothelial carcinoma
Age
Younger
Older
Sex (male/female)
2 : 1
3 : 1
Size
Small, usually <2 cm
Typically larger than papilloma
Microscopic findings
Well-formed papillae
Present
Present, rarely fused
Thickness of urothelium
≤7 layers
>7 layers
Superficial umbrella cells
Present
Usually present
Cytology
Minimal or absent
Mild
Nuclear enlargement
Rare or none
None or slightly enlarged
Nuclear hyperchromasia
Rare or none
Slight or minimal
Chromatin
Fine
Fine, slightly granular
Nucleolar enlargement
Absent
Absent or inconspicuous
Nuclear pleomorphism
Absent
Absent
Mitotic figures
None
Rare or basal location
Stromal invasion
Absent
Rare
Grade 2 urothelial carcinoma
Grade 3 urothelial carcinoma
Histologic grading according to the International Society of Urological Pathology 1998 and World Health Organization 2004 classifications
Papillary urothelial neoplasm of low malignant potential
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree