Fig. 4.1
Retrograde pyelogram showing a left ureteropelvic junction (UPJ) obstruction from a high insertion of the left ureter
When intervention is indicated, the reference standard has historically been open dismembered pyeloplasty [5]. However, with the advancements in endourological instrumentation, the direction has shifted toward minimally invasive procedures such as endopyelotomy (antegrade and retrograde), laparoscopic pyeloplasty, and robotic pyeloplasty [6]. All minimally invasive procedures share the advantage of short operative time, minimal morbidity, decreased postoperative analgesia, shorter hospital stay, and early recovery [7]. Still, a few questions remain. Which patients should undergo ureteroscopy as the first-line treatment? Are antegrade and retrograde approaches comparable in treatment success? What is the role of endopyelotomy among other minimally invasive procedures? What are the exclusion criteria for endopyelotomy? This chapter focuses on the techniques and clinical results for ureteroscopic endopyelotomy for the management of UPJO.
History of Retrograde Approach
The initial concept behind the endourological approach to UPJO can be traced back to Davis and colleagues in 1943 [8]. After a full-thickness incision of the narrow ureteral segment, a stent is placed to allow regeneration of an adequate caliber ureter around the stent. In 1983, Wickham and Kellet described the first ureteroscopic pyelolysis of the UPJ [9]. Bagley et al. reported their combined percutaneous and flexible ureteroscopic procedure approach for the management of an obliterated UPJ in 1985 [10]. As percutaneous renal surgery became more commonplace in the 1980s, antegrade endopyelotomy was introduced by Badlani et al. in 1986 [11] with good success. The retrograde approach became the next logical evolution of the minimally invasive approach in the treatment of UPJO.
The rationale for the introduction of a retrograde approach for endopyelotomy was to make the procedure less invasive by avoiding the potential complications associated with percutaneous renal access. Retrograde endopyelotomy performed with a rigid ureteroscope and electrocautery hook was first reported by Inglis and Tolley in 1986 [12]. The Acucise cutting balloon catheter was then approved by the FDA in 1993 in an effort to simplify retrograde endopyelotomy. This device made it possible for all urologists to perform retrograde endopyelotomy under fluoroscopic control. Thomas et al. [13] reported their experience with pre-stenting to facilitate ureteroscopic endopyelotomy in 1996. Subsequently, a one-stage procedure was described by Soroush and Bagley in 1998 [14]. Failed endopyelotomy did not jeopardize the success of subsequently performed open pyeloplasty [15]. Therefore, endopyelotomy was considered the first-line treatment of UPJO in appropriate clinical situations [6]. Further experience revealed that primary cases of UPJO could be successfully treated [16].
Diagnosis and Preoperative Evaluation
When there is clinical suspicion of UPJO, subsequent radiographic studies should always be performed to confirm the diagnosis. Proper imaging can determine both the anatomic site and the functional significance of obstruction. Renal ultrasound, retrograde pyelography, IVP, diuretic renal scan, Whitaker test, or a combination of them can be used. The diuretic renal scan not only verifies the presence of an obstruction, but also provides quantitative differential renal function, which can direct the selection of the best treatment option. Furthermore, the renal scan facilitates follow-up evaluation of the renal function after treatment. CT angiography (Fig. 4.2) is a common imaging modality, since it provides spatial anatomical information, specifically that of aberrant crossing vessels. A combination of anatomic and functional studies, such as retrograde pyelography and diuretic renal scan, can sometimes be utilized to best plan treatment. For example, retrograde pyelography can both confirm and demonstrate the exact site and nature of obstruction before repair. In most instances, this test is performed at the time of the planned intervention in order to avoid the risk of introducing infection in the setting of obstruction. In the setting of infection or compromised renal function, however, retrograde pyelography and stent placement are indicated emergently for decompression of UPJO. The UPJ can even be evaluated ureteroscopically for pulsations at the time of endopyelotomy. This evaluation can be aided by the use of endoluminal ultrasound [17]. When scheduling operative intervention, risks and benefits of all treatment techniques should be discussed with the patient, since the indications for intervention for UPJO are similar regardless of technique. All the anatomic and functional information should be thoroughly reviewed preoperatively, and all treatments should be individualized. Patients should also be informed that sometimes a secondary intervention may be necessary.
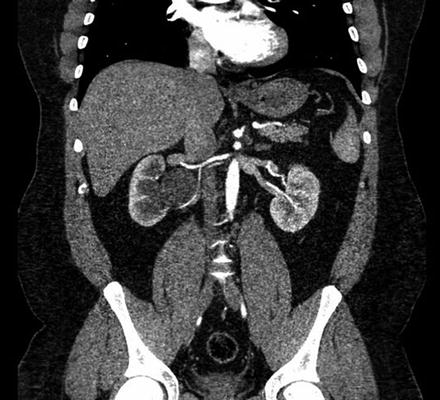

Fig. 4.2
CT angiography demonstrating a right ureteropelvic junction (UPJ) obstruction with a crossing vessel
Patient Selection
Although historical success rates with retrograde endopyelotomy have been 10–15% lower than those of open, laparoscopic, or robotic pyeloplasty, it has been noted that success rates can be greatly improved with careful patient selection [16]. Van Cangh et al. reported an overall success rate for endopyelotomy of 73%. However, when patients with both a crossing vessel and a high degree of obstruction were excluded from analysis, the success rate improved to 95%, which is comparable with that of open pyeloplasty [18]. Thus, in an appropriately chosen patient cohort, endopyelotomy still can be a significant and successful minimally invasive option in the treatment of UPJO.
Absolute contraindications include active urinary tract infection, sepsis, and uncontrolled coagulopathy. Relative exclusion criteria are based on a definitive set of clinical parameters that strongly correlate with success rate. Massive hydronephrosis (grade 4), stenosis greater than 2 cm and preoperative ipsilateral renal function <20% have been associated with poor endopyelotomy outcomes [15]. Tan and Smith also noted that total obliteration of the UPJ was also linked with unsatisfactory results [16]. Finally, patients with concurrent renal stones and UPJO and patients with a nephrostomy tube in place should be excluded from the retrograde approach. In these instances, antegrade endopyelotomy should be performed to address both the renal stones and the UPJO in one-stage procedure [16]. Berkman et al. showed that stone removed at the time of antegrade endopyelotomy did not offset success. Overall success rate was reported in 71% of patients undergoing endopyelotomy only and 90% of 41 patients who had simultaneous nephrolithotomy. Also, when adjusting for the severity of preoperative hydronephrosis and/or renal function, simultaneous nephrolithotomy did not decrease the success rate [19]. Patients with poor intrinsic renal function and severely decreased parenchyma should be initially drained by a stent and reevaluated or should be offered a laparoscopic simple nephrectomy for a poorly functioning or nonfunctioning kidney. Patients with massive hydronephrosis, but adequate function (>10–20%), should be treated with dismembered pyeloplasty, either open, laparoscopically, or robot assisted, because of the need for trimming and reduction of the redundant renal pelvis. The presence of massive hydronephrosis can also influence which approach, antegrade or retrograde, should be employed for endopyelotomy. Lam et al. reported that antegrade endopyelotomy was more successful than retrograde endopyelotomy in patients with massive hydronephrosis (66.7 vs. 20.0%) with equal minimal morbidity [20].
Controversy still exists with patients with high insertion of the UPJ in proceeding with retrograde endopyelotomy. Although once considered a contraindication due to initial poor results, there are subsequent published series that documented that high ureteral insertion had no significant impact on the outcome of endopyelotomy [15]. Alternatively, open, laparoscopic, or robotic-assisted pyeloplasty in the case of a known crossing vessel can be offered as a primary treatment modality.
In the past, when the decision was made to proceed with ureteroscopic management of UPJO, a preoperative ureteral stent was placed to drain the obstruction for 1–2 weeks. This maneuver accomplished several important goals. First, it delineated improvement of renal function after drainage and assessed the patient’s ability to tolerate an indwelling stent. The latter is an important distinction, given that after retrograde endopyelotomy a stent is typically left indwelling for 6 weeks. Moreover, the stent dilated and straightened the UPJ, stabilized renal function, and facilitated the passage of an ureteroscope at the time of endopyelotomy. Now with smaller, flexible ureteroscopes and laser fibers, this extra step is not necessary and a one-stage procedure can be performed safely and effectively with less morbidity.
Technique
Currently, retrograde ureteroscopic endopyelotomy can be performed in three ways: (1) using a rigid ureteroscope and a cold-knife, electrocautery, or holmium laser incision; (2) using a flexible ureteroscope and electrocautery or laser incision; and (3) using a cutting balloon catheter (Acucise, Applied Medical Resources Corporation, CA). After either general or a spinal anesthesia, the patient is placed in the lithotomy position. Caution is taken so that all pressure points are well padded, and preoperative antibiotics are given.
As ureteroscopic technique has evolved over time, laser energy has largely replaced electrocautery to incise the UPJ. High energy produced by laser provides instant vaporization of tissue for more precise incision, in contrast to low energy produced by electrocautery leading to tissue coagulation and damage to peripheral blood supply. Moreover, the small diameter and flexibility of laser fibers enable their passage through small-diameter semirigid and flexible ureteroscopes, providing more degrees of freedom [6, 21].
Ureteroscopic Endopyelotomy with Electrocautery
Due to recent advancements in laser technology, the electrocautery technique is not utilized as frequently and will be discussed briefly. In one technique, a 5 F open-ended catheter is placed over a superstiff wire to maintain renal access while preventing conduction of electrical current as with a standard guidewire (Figs. 4.3 and 4.4). The catheter can also serve to continuously drain the renal pelvis of irrigation fluid during the procedure. The ureteroresectoscope is then passed directly alongside the catheter [1]. If there is difficulty accessing the ureteral orifice or ureteral narrowing, these portions can be balloon dilated. Either water or 1.5% glycine solution should be used for irrigation. The UPJ is first inspected for transmitted pulsations and then incised laterally, between 8 and 9 o’clock on the right and between 3 and 4 o’clock on the left [1]. Short, superficial strokes should be performed to avoid bleeding. Any bleeding should be controlled with spot electrocoagulation. A full-thickness incision is carried down to periureteral fat, and performed widely enough so that the scope can easily enter into renal pelvis.

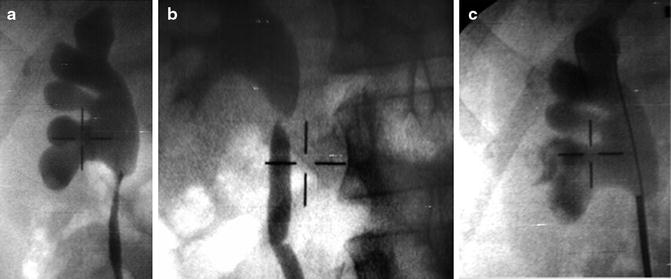

Fig. 4.3
Rigid ureteroscope

Fig. 4.4
(a) Intraoperative fluoroscopy (a) showing ureteropelvic obstruction, (b) with safety wire placement, and (c) confirming position of rigid ureteroscope at the ureteropelvic stenosis
Ureteroscopic Laser Endopyelotomy
Two types of lasers have been described in literature when performing ureteroscopic laser endopyelotomy: neodymium:yttrium aluminum garnet (Nd:YAG) and holmium:YAG (Ho:YAG). The Nd:YAG, with a wavelength of 1,060 μ(mu)m, produces a wider scatter and deeper penetration into tissue than the holmium:YAG. Initial success rate of 85% using the Nd:YAG laser was reported by Renner et al. [22], but a recurrence UPJ stenosis rate of 45% was observed by Gallucci et al. [23]. Potential reasons for high recurrence rate lie in the nature of Nd:YAG laser, which relies primarily on thermal coagulation, necrosis, and delayed tissue sloughing. Thus, Biyani et al. concluded that the Nd:YAG was not ideal for laser endopyelotomy [24]. On the contrary, the depth of penetration of the Ho:YAG laser is less than 0.5 mm because of its longer wavelength of 2,100 μ(mu)m. Additionally, tissue absorption of the Ho:YAG laser is more than that of Nd:YAG laser because the Ho:YAG is absorbed by water. These characteristics lead to a rapid rise in tissue temperature which results in prompt vaporization upon tissue contact and coagulation upon near-contact. With the Ho:YAG laser endopyelotomy, a precise incision can be performed with minimal bleeding, because it possesses both ablative and hemostatic properties. Controlled endoscopic incision can be performed because the depth and direction of the incision can be better controlled without scattering tissue [24].
Typically, a 200–300 μm fiber is used with the flexible ureteroscope, while a bigger 365 μm fiber can be used through the semirigid scope. Power setting is set from 0.5 to 1.5 J at the rate of 5–10 Hz when making a full-thickness, lateral, or posterolateral incision (Figs. 4.5 and 4.6). The laser vaporization is started just below the UPJ and advanced proximally through the stenosis until the renal pelvis is reached. All layers are incised until encountering periureteral and peripelvic fat (Fig. 4.7). For hemostasis, the laser beam can be defocused (firing near but without contacting the tissue) to control minor bleeding from the renal pelvis. After the full-thickness incision, subsequent balloon dilation can further ensure complete incision of UPJO, but is not necessary. A double-J ureteral stent is always left in place for 4–6 weeks (Figs. 4.8 and 4.9) [24].
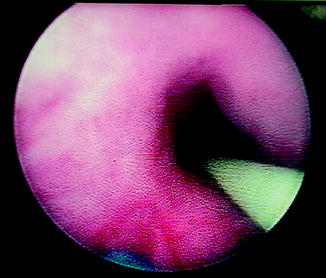
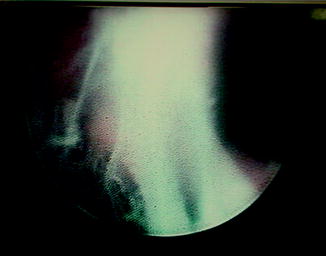
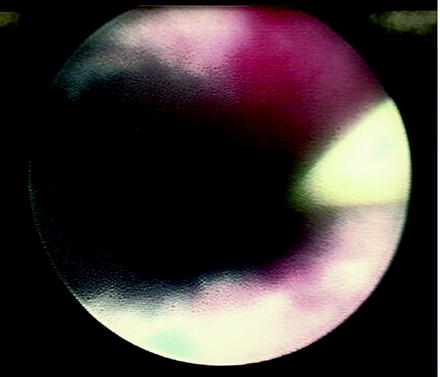
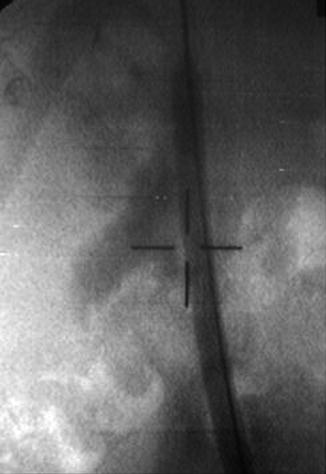
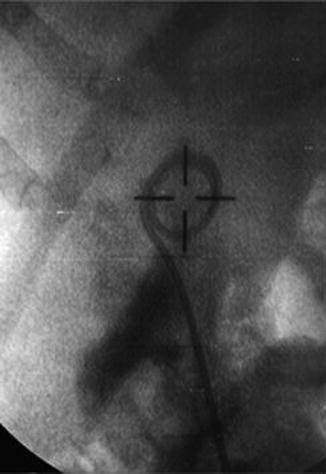

Fig. 4.5
Endoscopic view of ureteropelvic junction (UPJ) obstruction during retrograde laser endopyelotomy. Note the placement of a safety wire and the posterolateral location of the laser fiber during incision

Fig. 4.6
Adventitial fibers noted during laser endopyelotomy. These fibers must be completely taken down to ensure a full-thickness incision

Fig. 4.7
Full-thickness incision shown during laser endopyelotomy

Fig. 4.8
Intraoperative fluoroscopy confirming extravasation after laser endopyelotomy

Fig. 4.9
An indwelling double-J stent placement after laser incision of ureteropelvic junction obstruction
Cutting Balloon Catheter Endopyelotomy
The cutting balloon catheter (Acucise, Applied Medical Resources Corp., CA) features a monopolar electrocautery wire joined with a low-pressure balloon (Fig. 4.10). The balloon is used to define the area of stenosis; radio-opaque markers located on the proximal and distal end of the balloon assist in positioning the cutting wire during the incision. This device was initially developed as a 7 F catheter, but 5 F model was later developed which made preoperative stenting optional. Of note, high-quality fluoroscopic imaging is critical for optimal position of the cutting wire. Before the insertion through the ureteric orifice, the catheter is rotated so that the cutting wire is positioned laterally. Although not an absolute contraindication, the presence of crossing vessels warrants extreme caution with this technique. Evaluation with preoperative CT with angiographic phase is critical in minimizing bleeding complications. Under fluoroscopic guidance, the Acucise catheter is advanced over a guidewire toward the UPJ (Fig. 4.11). The ***balloon is initially inflated with 0.5–1 ml of diluted contrast to ensure correct position demonstrated by characteristic “waist.” The monopolar wire is then activated at 75–100 W of pure cut for about 5 s, while additional 2–3 ml of diluted contrast is simultaneously instilled into the balloon. As the balloon inflates, the stricture is incised, noted by the disappearance of the waist of the stricture (Fig. 4.12). If the waist persists, the cutting wire can be reactivated for additional 5 s. After a presumed incision, the inflation of balloon is maintained for about 5–10 min to tamponade any bleeding. The balloon is deflated, and retrograde pyelography is performed through the catheter to confirm extravasation at the incision site (Fig. 4.13). If extravasation is not seen, the UPJ can be directly inspected by a subsequent ureteroscopy. Periureteral fat should be visualized to ensure full-thickness incision (Fig. 4.14). Once this is confirmed, a ureteral stent is placed over the guidewire, transversing the site of UPJ incision. A Foley catheter is then placed for 24–48 h to decompress the bladder. The stent is left in place for 4–8 weeks [25].