1. Tumour located in the bladder dome
2. Predominant invasion of the muscularis or deeper with a sharp demarcation between the tumour and surface bladder urothelium that is free of glandular or polypoid proliferation
3. Absence of cystitis cystica and cystitis glandularis
4. Presence of urachal remnants within the neoplasm
5. Ramifications of tumour in the bladder wall with extension to the cave of Retzius, anterior abdominal wall or umbilicus
Other malignant tumours of urachus include sarcomas (leiomyosarcoma, rhabdomyosarcoma and malignant fibrous histiocytoma), small cell carcinoma, urothelial carcinoma and mixed types.
Staging
Currently there is no standard staging system for UrC but the most commonly accepted system being that of Sheldon [2] (Table 38.2).
Table 38.2
Sheldon staging of urachal cancer
Stage I | No invasion beyond mucosa |
Stage II | Invasion confined to urachus |
III | Local extension |
A | Bladder |
B | Abdominal wall |
C | Peritoneum |
D | Extension into viscera other than bladder |
IV | Metastases |
A | Lymph nodes |
B | Distant sites |
Clinical Presentation
Urachal cancer has an insidious course and when it presents with symptoms and signs it is normally of an advanced stage. It tends to be found more commonly in male patients with the largest series reporting a 2:1 ratio of men to women [1, 7–9]. Haematuria remains the most common mode of presentation in the great majority of cases being present in over 80 % of cases [1, 7]. Other symptoms and signs include suprapubic pain, lower urinary tract symptoms, mucosuria, umbilical discharge, urinary tract infection and abdominal mass.
Investigations
Because of its different behaviour compared to urothelial cancer it is important to recognise the condition as early as possible. It has also been moderately correlated with advancing pathological grade [7]. Cystoscopy will reveal a midline mass anteriorly in most cases and a biopsy should be taken. A negative cystoscopy however does not exclude an urachal tumour. Cross sectional imaging with CT or MRI will invariably reveal a mass, thickening or calcification at the bladder dome. This will also allow for accurate staging with respect to local extension, lymph nodes and distant metastasis.
Surgical Treatment
The primary surgical treatment of urachal cancer has varied in the extent of surgical resection with debate as to whether radical cystectomy or partial cystectomy were the preferred treatment options. The evidence from the literature would suggest that optimal treatment is total urachectomy. Henly et al. [10] reported their 34 cases treated with partial or radical cystectomy – 30 and 4 cases respectively. All of their cases underwent urachectomy and overall they demonstrated 43 % 5-year survival [10]. The experience from the MD Anderson noted that only 19 of 35 underwent urachal resection and it was noted that they were the majority of the long term survivors as compared with those who did not undergo urachectomy [11]. Ashley et al. [7] also analyzed the cases from the Mayo clinic and 60 cases were treated primarily with surgery but only 27 underwent urachectomy. Despite this overall survival was 48 % however they noted that that complete urachectomy and umbilectomy were significant predictors of survival on univariate analysis. In summary therefore it is recommended that complete urachectomy is undertaken which includes taking bladder dome and all the urachal remnants. There is no survival advantage of radical cystectomy over partial cystectomy as long as total urachectomy has been performed and surgical margins are clear [10, 12].
Chemotherapy
There is a limited role for chemotherapy inferred from the small amount of experience available. Logothetis et al. [13] used a combination of 5-fluorouracil, doxorubicin and mitomycin on 3 patients with urachal cancer. Two of the patients had a partial response and even then the response was short lived [13]. At MD Anderson [11] 29 patients were treated with chemotherapy – 20 for metastatic disease, 7 received postoperative adjuvant treatment for margin or node positive disease and 2 had neoadjuvant therapy. The objective response rate was 33 % in 9 patients who had a regimen that combined 5-fluorouracil and cisplatin with either α-interferon or gemcitabine and leucovorin. The median survival in metastatic disease was 20 months and chemotherapy did not appear to make any difference to outcome apart from some objective responses. Similar findings with chemotherapy usage were reported from the Mayo Clinic with no differences in time form metastases to death [7]. Their chemotherapeutic agents were cisplatin (50 % of patients), doxorubicin (36 % of patients), cyclophosphamide (21 % of patients), 5-fluorouacil (21 % of patients), and paclitaxel (14 % of patients). A recent trial of 5-fluorouracil, leucovorin, gemcitabine and cisplatin has completed a study on metastatic urachal carcinoma with patients being followed up [14].
Radiation has had little role in urachal cancer and primarily used as adjuvant therapy in disease recurrence and loco-regional symptom control. The little use it has had as primary therapy was associated with decreased survival compared with those surgically treated although this may have been due to the patients irradiated being older and having higher stage disease [7].
Prognosis
The prognosis for urachal cancer remains poor with its insidious course and advanced disease at presentation. Factors associated with a poorer prognosis include positive surgical margins, failure to complete total urachectomy, radiation therapy as primary treatment, increasing tumour grade and stage [7, 11, 12, 15].
Urethral Cancer (UC) (Excluding Penile Cancer)
Primary neoplasm of the urethra is rare which makes its study difficult with a lack of studies in the literature – the ones reported are retrospective reviews of treatment. Therefore it has not been possible to adequately define the natural history or suggest an ideal management strategy in these tumours. At presentation it has often invaded locally contributing to its generally poor prognosis despite the treatment modality employed. This section will primarily deal with primary malignancy of urethra with specific reference to transitional cell carcinoma.
Epidemiology and Risk Factors
Primary urethral cancer is extremely rare accounting for less than 1 % of all malignancies. Female urethral cancer is four times more common than male urethral cancer. It typically presents after the age of 60. Primary carcinoma of the urethra accounts for only 0.02 % of all malignancies in females [16]. The exact aetiology is not known. Caucasians seem to have a higher incidence [17]. There are number of differences between male and female urethral malignancy due to anatomic and histological factors.
Risk Factors
Like many other cancers chronic inflammation seems to play a role in the pathogenesis of urethral cancer. In males, an increased incidence of primary urethral cancer has been associated with sexually transmitted diseases and urethral stricture disease [18]. UC has been reported in patients with intermittent catheterization/ urethroplasty [19], external beam irradiation therapy /radioactive seed implantation [20], and chronic urethral inflammation/urethritis following sexually transmitted diseases (i.e. condylomata associated with human papilloma virus 16) [21, 22]. Clear cell adenocarcinoma may also have a congenital origin [23]. There is also evidence to suggest that human papilloma virus 16 (HPV-16) is associated with the development of squamous cell cancer of the urethra [21]. For females these associations are not so strong but chronic irritation and urinary tract infections have been implicated with urethral cancer.
Other factors quoted include pre-existing lesions such as caruncles, diverticula, papillomas, adenomas, polyps and leukoplakia of the urethra [24]. Smoking is a risk factor for bladder cancer, as well as a risk factor in the development of transitional cell urethral carcinoma.
Anatomy
The anatomy of the urethra explains the pathological types, progression and behavior of urethral carcinoma in both sexes.
Male Urethra
This has an approximate length of 20 cm. For descriptive purposes the male urethra is anatomically divided into Penile, Bulbar, Membranous and Prostatic segments (Fig. 38.1 and Table 38.3).
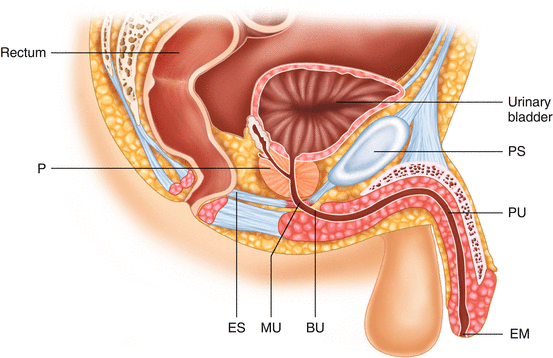

Fig. 38.1
Diagrammatic representation of urethra and bladder. ES external sphincter, BU bulbar urethra, PU penile urethra, P prostate, EM external urethral meatus, MU membranous urethra, PS Pubic symphisis
Table 38.3
Urethral segments and epithelial type in the male
Urethral segment | Epithelium |
|---|---|
Urethral meatus, fossa navicularis | Stratified squamous |
Penile, bulbar, | Pseudostratified and stratified columnar |
Prostatic, membranous | Transitional |
The male urethra may be sub-divided into ‘anterior’ and ‘posterior’ according to the lymphatic drainage in men. The anterior urethra comprises the urethral meatus, fossa navicularis and penile segment and their lymph drainage is to the inguinal nodes.
The posterior urethra comprises of bulbar, membranous and prostatic segments, which drain to the pelvic nodes.
Female Urethra
The female urethra is shorter than its male counterpart with an approximate length of 4 cm. The distal two-thirds are lined by stratified squamous epithelium while the proximal third comprises transitional cell epithelium. The distal third or anterior urethra drains to the superficial and deep inguinal lymph nodes. The proximal two-thirds or posterior urethra drains to the pelvic nodes.
Pathology
Male
Benign Tumours
Condyloma acuminatum (syn genital or venereal warts) (Human papilloma virus Type 6–11); slow growing; Urethral involvement in 5 % of patients; they are commonly seen on the glans, shaft and prepuce.
Urethral polyps: fibrous (usually congenital and early presentation) or prostatic polyps (uncertain etiology and related reactive proliferations secondary to urethral injury). The lesion could be sessile or papillary.
Premaligant
Balanitis xerotica obliterans (BXO): genital variation of lichen sclerosis et atrophicus.
Leukoplakia are whitish plaques involving the meatus and associated with in situ squamous carcinoma and verrucous cancer.
Carcinoma in situ (Syn. Erythroplasia of Queyrat or Bowen’s disease of glans) [25].
Malignant Tumours
Primary urethral malignancy accounts for less than 1 % of genito-urinary tumours in men. The common histological varieties include squamous (80 %), urothelial (15 %), adenocarcinoma (5 %) and rarer varieties including malignant melanoma, basal cell carcinoma and sarcomas. The commonest urethral tumor, squamous cell carcinoma occurs in bulbo-urethral region (60 %). Ninety percent of cancers in the prostatic urethra are transitional in origin reflecting the predominant epithelial type in this location.
Female
In a series of 18 female patients Thayavihalli et al. [30] found the predominant histology to be squamous cell cancer accounting for 50 % of cases. Transitional cell cancer accounted for 28 % and adenocarcinoma 22 %.
Clinical Presentation
There is no typical clinical presentation of urethral cancer. Because of the early local involvement most of the tumours are advanced at the time of presentation. The presentation is insidious and the symptoms are often ascribed to benign disorders. The most common presentation is with a palpable urethral lump or with lower urinary tract symptoms. Alternative presentations include urethral stricture disease, urethral fistulation, urethral diverticula, abscesses, recurrent urinary tract infections, dyspareunia, perineal pain and lymphadenopathy. Haemospermia may be an additional symptom in men. It must be emphasized that a strong index of suspicion should be exercised with a low threshold for investigation so that any delay is avoided. In patients who have undergone urethra-sparing cystectomy urethral bleeding is the only sign of urethral TCC. However regular endoscopic surveillance is mandatory in these cases.
TNM Staging
It spreads locally to the corpora, periurethral muscle and adjacent organs including vagina, prostate, bladder and rectum (Table 38.4). Palpable lymph nodes are present in 20 % of patients at presentation and most of them will have tumour within these nodes. Haematogeonous spread is rare and a late event.
Table 38.4
TNM classification of urethral cancer
Primary | Tumour |
|---|---|
Local tumour | |
Tx | Primary tumour cannot be assessed |
T0 | No evidence of primary tumour |
Ta | Non-invasive papillary, polypoid or verrucous carcinoma |
Tis | Carcinoma-in-situ |
T1 | Tumour invading subepithelial connective tissue |
T2 | Tumour invading any of the following: corpus spongiosum, prostate, periurethral muscle |
T3 | Tumour invading any of the following: corpus cavernosum, beyond prostatic capsule, anterior vagina, bladder neck |
T4 | Tumour invades other adjacent organs < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




