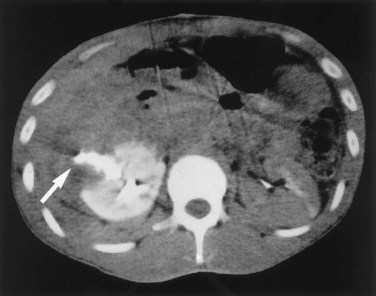
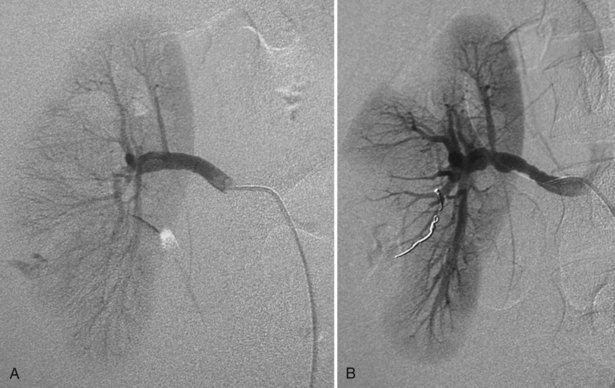
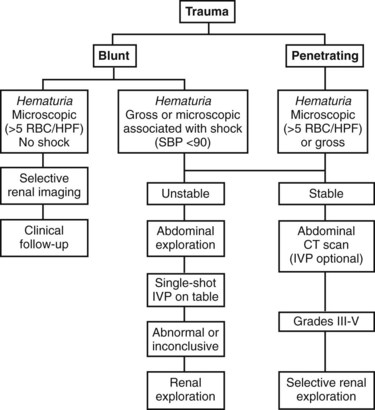
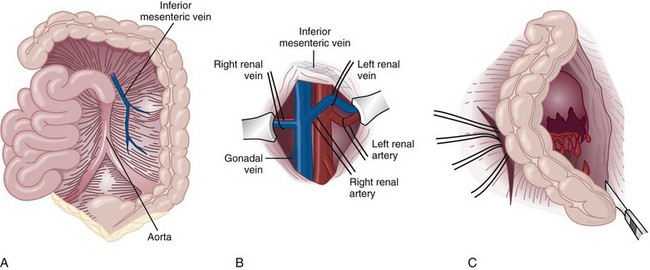
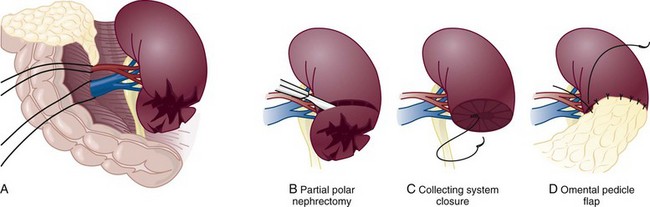
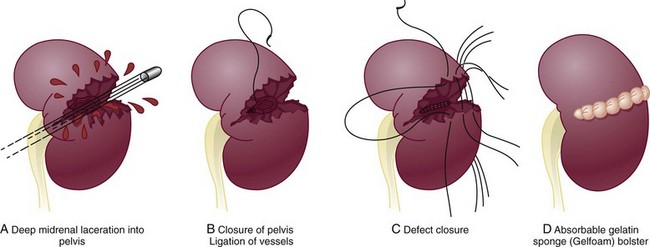
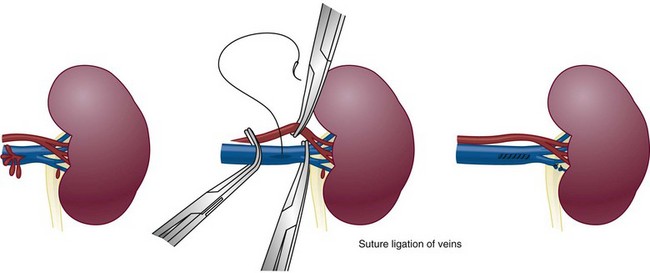
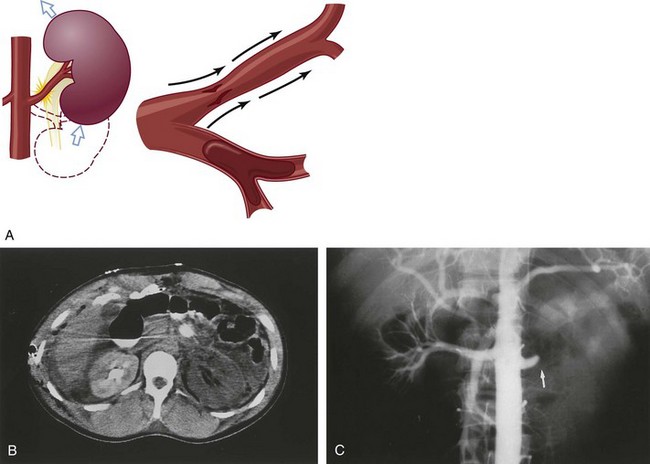
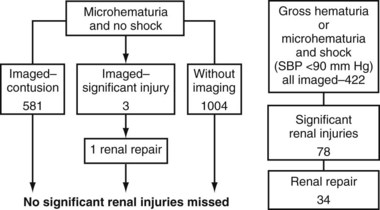
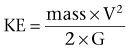Richard A. Santucci, MD, Leo R. Doumanian, MD Traumatic injury is a leading national and international health problem. In the United States, 1 of every 14 deaths—more than 150,000 per year—results from trauma (Baker et al, 1992). Indeed, in young people, trauma results in more deaths between ages 1 and 37 years than any other cause. Violence alone claims 50,000 lives annually, and more than 2.2 million in the United States suffer injuries from assaults each year. With the development of trauma care systems and centers, death rates from major injuries have declined significantly over the past 20 years (Kivioj et al, 1990). The first hour of care after a major injury is extremely important and requires rapid assessment of the injuries and resuscitation based on priorities established by the American College of Surgeons’ Acute Trauma Life Support Program. The mnemonic “ABCDE” defines these priorities in order of importance: A, airway with cervical spine protection; B, breathing; C, circulation and control of external bleeding; D, disability or neurologic status; and E, exposure (undress) and environment (temperature control) (American College of Surgeons Committee on Trauma, 2004). Penetrating renal injuries most often come from gunshot and stab wounds. Gunshot wounds comprise the great majority of penetrating trauma with stab wounds a distant second (86% vs. 14%). Injuries to the upper abdomen or lower chest should immediately alert the physician to potential renal injury. Often presenting with a more advanced grade of injury, penetrating mechanisms lead to higher rates of surgical exploration. Of all patients sustaining renal trauma in a large reported series, renal gunshot wounds occurred in approximately 4% (McAninch et al, 1993). where KE = kinetic energy, V = velocity, and G = gravity. Whether the preceding equation accurately reflects tissue destruction is controversial; however, the result of extensive soft tissue damage caused by high-velocity weapons requires debridement guided by individualized clinical observation (Santucci and Chang, 2004). The greater the bullet velocity, the larger the temporary cavity created, and the larger potential for soft tissue stretch and destruction (Hutton and Rich, 1996). Handguns are generally considered low-velocity firearms (<2000 ft/sec). Other factors to consider are bullet shape (i.e., blunt or narrow), type (i.e., hollow point, exploding, frangible), caliber, and velocity, which all may independently contribute to tissue disruption and injury. The best indicators of significant urinary system injury include the presence of microscopic (>5 red blood cells/high-power field [RBCs/HPF] or positive dipstick finding) or gross hematuria and hypotension (systolic blood pressure <90 mm Hg). The presence of microscopic hematuria is often characteristic. However, gross hematuria has been observed in minor renal contusions and microscopic hematuria has been associated with severe renal injuries. For example, hematuria was absent in 7% of 420 grade IV renal injuries in a recent analysis (Shariat et al, 2008a) and 36% of renal vascular injuries from blunt trauma demonstrated no blood in the urine (Cass, 1989). Also, approximately 50% of injuries to the ureteropelvic junction have no microscopic or gross hematuria. Therefore the degree of hematuria and the severity of the renal injury do not consistently correlate. In patients with blunt trauma, if shock is noted with microscopic hematuria, the incidence of significant renal injuries increases (Mee et al, 1989; Miller and McAninch, 1995; Nicolaisen et al, 1985). In addition, hematuria has been used as a general predictor of nonrenal intra-abdominal organ injury after blunt trauma (Knudson et al, 1992). The authors use the first aliquot of urine obtained either by catheterization or by voiding to determine the presence of hematuria. Later urine samples are often diluted by diuresis from resuscitation fluids, resulting in an underestimation or absence of hematuria. Any degree of visible blood in the urine is regarded as gross hematuria. Microscopic hematuria can be detected by dipstick analysis or microanalysis. The dipstick method is rapid and has a sensitivity and specificity for detection of microhematuria of more than 97%, even though a poor correlation with actual urinalysis was noted in a study by Chandhoke and McAninch (1988). Although critical to the initial evaluation of traumatic urinary tract injury, the presence or absence of hematuria should not be the sole determinant in the assessment of a patient with suspected renal trauma. Because the significance of hematuria varies with blunt and penetrating mechanisms, the importance of proper staging of renal injuries cannot be underscored. Numerous models have been proposed for staging and management of renal trauma according to the severity of the injury. The American Association for the Surgery of Trauma’s Organ Injury Scaling Committee (Moore et al, 1989) developed the most widely used and accepted classification (Table 42–1; Fig. 42–1). On the basis of accurate grading made possible by contrast-enhanced computed tomography (CT), the AAST injury severity scale is a powerful and valid predictive tool for clinical outcomes in patients with renal trauma. Even with these minor variations in reporting, the classification system has established a common scale by which injuries can be identified at disparate centers according to the extent of damage, and it has worked well (Santucci et al, 2001). The widespread use and anatomic detail provided by CT imaging has now supplanted the much less sensitive and less specific excretory urography (intravenous pyelography [IVP]) for grading purposes. Table 42–1 American Association for the Surgery of Trauma Organ Injury Severity Scale for the Kidney * Advance one grade for bilateral injuries up to grade III. Data from Moore EE, Shackford SR, Pachter HL, et al. Organ injury scaling: spleen, liver, and kidney. J Trauma 1989;29:1664–6. The best indicator of renal injury is hematuria, defined as 5 RBCs/HPF by most authors. Historically, when used as the sole indication for renal imaging, IVP and other studies found a low incidence of renal abnormalities. An extensive prospective study based at San Francisco General Hospital evaluating indications for radiographic imaging has been ongoing for more than 25 years. The findings have been updated on three reports (Mee et al, 1989; Miller and McAninch, 1995; Nicolaisen et al, 1985). Figure 42–2 provides the results of the study for adult blunt renal trauma. On the basis of information from this study, all blunt trauma patients with gross hematuria and patients with microscopic hematuria and shock (systolic blood pressure <90 mm Hg any time during evaluation and resuscitation) should undergo renal imaging, usually with CT using intravenous contrast. (From Miller KS, McAninch JW. Radiographic assessment of renal trauma. Our 15-year experience. J Urol 1995;154:352–5.) Patients with microscopic hematuria without shock can be observed clinically without imaging studies. As first noted by Miller and McAninch (1995), several studies confirm the findings that these patients rarely have a significant injury (<0.0016%). However, if possible renal injury is suspected on the basis of history or examination, imaging should be performed. For example, blunt rapid deceleration injuries such as high-speed motor vehicle accidents or falls from great heights are at risk for vascular injury. These can cause significant injury (usually ureteral avulsion or vessel injury) in the absence of microscopic hematuria. Penetrating injuries with any degree of hematuria should be imaged. In a report by Carroll and McAninch (1985), 27 of 50 patients with penetrating renal trauma had microscopic hematuria. Three of these patients had 0 to 3 RBCs/HPF, and one of the three had a renal pedicle injury. The presence of shock, degree of hematuria, location of the entry wound, and type of injury do not permit reliable discrimination among categories. Pediatric patients (younger than 18 years) sustaining blunt renal trauma must be carefully evaluated. Children are known to be at a greater risk for renal trauma than adults after blunt abdominal injury (Brown and colleagues, 1998a). For pediatric patients, liberal use of imaging studies should be considered, especially in the setting of high-speed/high-energy deceleration injuries (Buckley and McAninch, 2004). Children have a high catecholamine output after trauma, which maintains blood pressure until approximately 50% of blood volume has been lost. A recent review confirmed that the decision to image pediatric trauma cases on the basis of the adult criteria of gross hematuria, shock, and significant deceleration injury appropriately identified potential renal injuries just as they would in adult patients (Santucci et al, 2004). Contrast-enhanced computed tomography (CT) is the gold standard for genitourinary imaging in renal trauma (Bretan et al, 1986; Federle et al, 1987). Highly sensitive and specific, CT provides the most definitive staging information: Parenchymal lacerations are clearly defined; extravasation of contrast-enhanced urine can easily be detected (Fig. 42–3); associated injuries to the bowel, pancreas, liver, spleen, and other organs can be identified; and the degree of retroperitoneal bleeding can be assessed by the size and dimensions of the retroperitoneal hematoma. Lack of uptake of contrast material in the parenchyma suggests arterial injury. The added anatomic detail for diagnosis of renal contrast extravasation and parenchymal/vascular injuries has translated into improved confidence in our ability to nonoperatively manage major injuries. Currently, spiral CT is being used in many centers to evaluate renal injuries (Brown et al, 1998b). Arteriovenous scanning (typically 80 seconds after contrast administration) provides visualization of the kidneys in the nephrogenic phase of contrast excretion and is necessary to detect arterial extravasation. Injury to the renal collecting system may be missed; contrast material has not had time to be excreted into the parenchyma and collecting system adequately. Repeated/delayed scanning of the kidneys 10 minutes after injection of contrast identifies parenchymal lacerations and urinary extravasation accurately and reliably. Expert opinion holds that delayed films may be omitted when the kidneys are deemed normal, and no perinephric, retroperitoneal, pelvic, or perivesical fluid is present (Santucci et al, 2004). Findings on CT that raise suspicion for major injury are (1) medial hematoma, suggesting vascular injury; (2) medial urinary extravasation, suggesting renal pelvis or ureteropelvic junction avulsion injury; and (3) lack of contrast enhancement of the parenchyma, suggesting arterial injury. Morey and coworkers (1999) have reported their experience with single-shot intraoperative IVP for the immediate management of renal injuries; in 50 patients, the film quality was adequate to avoid renal exploration in 32%. This report supports the value of this intraoperative imaging technique when done properly. In select cases, renal arteriography can be considered an adjunct diagnostic imaging technique with possible therapeutic implications. Angiography is largely used to define arterial injuries suspected on CT or to localize and control arterial bleeding (Fig. 42–4). Renal embolization has proved useful in the primary setting with persistent bleeding in a hemodynamically stable patient. Case reports have shown that endovascular stenting can be used to treat traumatic renal artery dissection, but technical problems surrounding the need for postprocedure anticoagulation have not yet been solved. Pseudoaneurysms and arteriovenous fistula are treated by angiographic embolization to stop secondary hemorrhage. Superselective embolization therapy for renal trauma now provides an effective and minimally invasive technique to avoid unnecessary exploration that could otherwise result in a nephrectomy. Significant injuries (grades II to V) are found in only 5.4% of renal trauma cases (Miller and McAninch, 1995). Nonoperative management has become the standard of care in hemodynamically stable, well-staged patients with American Association for the Surgery of Trauma (AAST) grade I to III renal injuries, regardless of mechanism (Santucci et al, 2004). Most experts agree that patients with grade IV and V injuries more often require surgical exploration, but even these high-grade injuries can be managed without renal operation if carefully staged and selected (Fig. 42–5) (Santucci and McAninch, 2000; Santucci et al, 2004). A trial of expectant management has even been advocated for most adult blunt renal parenchymal injuries, many renal stab wounds, and selective renal gunshot wounds. Bluntly injured kidneys heal well when managed conservatively, even in the setting of urinary extravasation and nonviable tissue; 98% can be successfully managed without exploration (Broghammer et al, 2007). In fact, in a series of six hemodynamically stable, grade V blunt injuries, four (66%) kidneys demonstrated satisfactory function after conservative treatment (Altman, 2000). Penetrating trauma from gunshot or stab wounds to the kidney can also be managed nonoperatively in stable patients. In one large series, 55% of renal stab wounds and 24% of gunshot wounds were appropriately managed nonoperatively in carefully selected patients with well-staged injuries (McAninch et al, 1991). Contrary to past experience, obligatory exploration is no longer mandated for renal gunshot wounds. Serafetinides (2004) treated 40 patients (54%) with low-velocity gunshots expectantly with few complications. Stab injuries have more evidence to support conservative management. Nonoperative management protocols resulted in no delayed nephrectomies in a cohort of 108 hemodynamically stable stab wound patients (Armenakas et al, 1999) Although blunt and penetrating trauma often requires exploration due to associated nonurologic injury, many authors argue for continued conservative approach intraoperatively, particularly in the presence of an injured kidney with a nonexpanding, nonpulsatile hematoma (Shariat et al, 2008b). All patients with high-grade injuries (grades III to V) selected for nonoperative management should be closely observed with serial hematocrit readings. Strict bedrest is mandatory until gross hematuria resolves. Patients presenting with urinary extravasation or nonviable parenchyma may be considered for periodic inpatient imaging, although the authors are aware of no studies to prove it helps in the absence of worrisome symptoms (fever, flank pain, dropping hematocrit). Although most grades II to IV injuries resolve uneventfully, delayed renal bleeding can occur in up to 25% (Wessells et al, 1997). Should bleeding persist or delayed bleeding occur, angiography with selective embolization of bleeding vessels can obviate surgical intervention. The management of severe renal lacerations must balance two major issues: the increased incidence of nephrectomy in patients undergoing immediate as opposed to delayed renal exploration and the associated morbidity of patients managed expectantly. The experience with endovascular therapy for severe renal hemorrhage continues to grow. In a literature review of 10 case series, most renal traumas including most grade IV injuries were effectively managed with embolization therapy with a clinical success rate of 80% (Breyer, 2008). Indications for angiography with embolization therapy have included bleeding from a renal segmental artery with or without parenchymal laceration, unstable condition with grade III to IV injury, arteriovenous fistula or pseudoaneurysm, persistent gross hematuria, and/or blood loss exceeding 2 units in 24 hours. Future considerations to better define the criteria for angiography and embolization in patients with renal hemorrhage continue to be an area of research interest. In special clinical circumstances, endovascular stents have been used with reported success during angiography in patients with renal artery thrombosis occurring from intimal flaps (Goodman et al, 1998). Longer-term follow-up and more cases are necessary to determine whether this will be a successful management approach, especially considering that most stents require anticoagulation after placement, which may not be possible in a trauma patient. Those patients with grade IV parenchymal lacerations who have well-contained hematomas should be observed expectantly. An 88% renal salvage rate of isolated grade IV renal injuries managed conservatively has recently been documented (Buckley and McAninch, 2006). They should be closely monitored for bleeding, with vital signs, serial hematocrit readings, and pulse rates. If urinary extravasation is present, serial renal CT scanning can be considered. If significant urinary extravasation persists, placement of an internal ureteral stent for drainage often prevents prolongation of the extravasation and decreases the chance of perirenal urinoma formation. Experts recommend the use of broad-spectrum antibiotics to decrease the possibility of perinephric abscess, although the authors are aware of no convincing research that they decrease infection rates. Indications for renal exploration after trauma can be separated into absolute and relative (Voelzke and McAninch, 2008). Absolute indications include (1) hemodynamic instability with shock; (2) expanding/pulsatile renal hematoma (usually indicating renal artery avulsion); (3) suspected renal pedicle avulsion (grade 5); and (4) ureteropelvic junction disruption. Relative indications are now rare: urinary extravasation together with nonviable tissue, renal injury together with colon/pancreatic injury, and a delayed diagnosis of arterial injury (which most likely will need delayed nephrectomy). The management of the intraoperative presence of a nonexpanding retroperitoneal hematoma is controversial. Nonoperative therapy is recommended, regardless of mechanism. Urinary extravasation alone from a grade IV parenchymal laceration or forniceal rupture can be managed nonoperatively with an expectation of spontaneous resolution of more than 90%. Should nonviable tissue constitute more than 25% in association with a parenchymal laceration or urinary extravasation or both, the potential for complications greatly increases and operative management is recommended (Alsikafi et al, 2006). Segmental renal artery injury with an associated renal laceration results in a substantial amount of nonviable tissue (usually > 20%). Historically, it was believed that such injuries resolved more quickly with surgical reconstruction and tissue removal. Currently, nonoperative management is favored in hemodynamically stable patients, although concerns include delayed bleeding and urinoma formation. A recent series demonstrated excellent outcomes with only 1 of 18 (6%) patients requiring subsequent intervention during conservative management of segmental renal artery injuries (Elliott et al, 2007). Surgical exploration of the acutely injured kidney is best done by a transabdominal approach, which allows complete inspection of intra-abdominal organs and bowel. In some reported series of penetrating injuries, associated organ injury has been noted to be as high as 94% (McAninch et al, 1993). Injuries to the great vessels, liver, spleen, pancreas, and bowel can be identified and stabilized if necessary before renal exploration. The surgical approach to renal exploration is shown in Figure 42–6 (McAninch and Carroll, 1989). The renal vessels are isolated before exploration to provide the immediate capability to occlude them if massive bleeding should ensue when Gerota’s fascia is opened (Scott and Selzman, 1966). The transverse colon is lifted superiorly onto the chest, and the small bowel is lifted superiorly and to the right. This exposes the midretroperitoneum. An incision is made over the aorta in the retroperitoneum just superior to the inferior mesenteric artery. The incision is extended superiorly to the ligament of Treitz. Exposure of the anterior surface of the aorta is accomplished and followed superiorly to the left renal vein, which crosses the aorta anteriorly. With a vessel loop controlling the vein, the anatomic relationships of the right and left renal arteries as they leave the aorta provide the ability to isolate and secure these structures with vessel loops. The right renal vein can be secured through this incision; if this proves difficult, reflecting the second portion of the duodenum provides excellent exposure to the vein. Renal bleeding is a major cause of nephrectomy in renal trauma. Obtaining early vascular control before opening Gerota fascia can decrease renal loss: in a comparative series, the total nephrectomy rate was reduced from 56% to 18% (McAninch and Carroll, 1982). Carroll and coauthors (1989), evaluating the use of early vascular control, reported the need to occlude the vessels in 12% of renal explorations. In a series of 133 renal units in which early vessel isolation and control before opening Gerota fascia was achieved in all, McAninch and colleagues (1991) reported a renal salvage rate of 88.7%. The need for early vascular control has been questioned. Corriere and colleagues (1991) reported a series of renal units in which vascular control was obtained only if needed after opening Gerota fascia. In this group, the total nephrectomy rate was 37.1%. Atala and colleagues (1991) reported a similar group of patients with a total nephrectomy rate of 36.2%. On the whole, the currently available data support an improved renal salvage rate with early vascular control because patients who require temporary vascular occlusion cannot be reliably identified before renal inspection. The principles of renal reconstruction after trauma include complete renal exposure, measures for temporary vascular control, debridement of nonviable tissue, hemostasis by individual suture ligation of bleeding vessels, watertight closure of the collecting system if possible, coverage or reapproximation of the parenchymal defect, and judicious use of drains (Fig. 42–7). Renorrhaphy is illustrated in Figure 42–8 and involves exposure of the kidney, debridement of nonviable tissue, hemostasis obtained with absorbable 4-0 chromic sutures on bleeding vessels (the addition of hemostatic agents such as Floseal [Baxter; Deerfield, IL] may also be helpful), closure of the collecting system, and approximation of the margins of the laceration (3-0 absorbable suture) with the use of renal capsule and an absorbable hemostatic agent bolster such as Gelfoam (Pfizer; New York, NY). When polar injuries cannot be reconstructed, a partial nephrectomy should be done and all nonviable tissue removed, hemostasis obtained, and the collecting system closed. The open parenchyma should then be covered when possible by a pedicle flap of omentum (see Fig. 42–7). With its rich vascular and lymphatic supply, omentum promotes wound healing and decreases the risk of delayed bleeding and urinary extravasation. Should it not be available, the use of absorbable mesh, peritoneal graft, or retroperitoneal fat has been successful (Master and McAninch, 2006). Hemostatic agents such as Floseal are potent and have an increasing role in the management of genitourinary trauma (Evans and Morey, 2006). On the basis of experience from nephron-sparing surgery, gelatin matrix was applied to a porcine model of complex renal trauma and demonstrated less mean blood loss than conventional suture treatment (Hick et al, 2005; Pursifull et al, 2006). In a high percentage of major renal injuries, intra-abdominal structures are also injured, the liver and spleen being the most common. Injuries to the colon, pancreas, and stomach also occur frequently, and in previous years total nephrectomy was suggested because of the high complication rate with attempted renal salvage. However, renal repair in these injuries has been successful with minimal complications (Rosen and McAninch, 1994; Wessells and McAninch, 1996). Drains should be used liberally in these repairs. Renovascular injuries following trauma are uncommon and often have associated injuries requiring operative intervention. For major renovascular injuries, speedy nephrectomy is advocated. In rare instances where repair is possible, renal salvage rates are low, exemplified by a 33% renal salvage rate for main renal artery reconstruction even in the most expert of hands (Elliott et al, 2007). Vascular repair requires occlusion of the involved vessel with vascular clamps. The lacerated main renal vessels injured by penetrating trauma can be repaired with 5-0 nonabsorbable vascular suture (Fig. 42–9). Main renal artery thrombosis from blunt trauma occurs secondary to deceleration injuries. The mobility of the kidney results in stretch on the renal artery, which in turn causes the arterial intima, low in elastic fibers, to disrupt. The consequent thrombus occludes the vessel, rendering the kidney ischemic (Fig. 42–10). Prompt diagnosis by CT or angiography may lead to immediate renal exploration in the appropriate candidate in an attempt to salvage the kidney, but outcomes for salvage remain dismally low and nephrectomy is almost always required. Case reports of successful renal revascularization through the use of endovascular stents during angiography offer a new and perhaps promising approach to the problem of blunt trauma renal artery thrombosis caused by the intimal flap (Inoue et al, 2004). The great disadvantage of this approach has been the inability to effect anticoagulation in the polytrauma patient. With delayed diagnosis (>8 hours), the kidney typically cannot be salvaged (Cass, 1989). With a 15-year experience, Haas and colleagues (1998) have reported that surgical revascularization was seldom successful in renal artery thrombosis and at least 43% of their patients with repairs developed hypertension. Hypertension was also common in those observed nonoperatively. When repair is attempted in renal artery thrombosis, the area of injury (noted by a visible contusion on the vessel) should be excised and a replacement graft done, preferably with hypogastric or splenic artery. Many patients with renal vascular injury are critically injured, with numerous associated organ injuries; time constraints thus limit attempts at vascular repair and a nephrectomy must be done. Injuries to the main renal vein require repair with fine vascular suture (5-0) (see Fig. 42–9). Partial occlusion of the vein is ideal for repair, but in some instances total occlusion with vascular clamps is necessary; if so, temporary occlusion of the renal artery is necessary. Coburn (2002) has noted the benefit of damage control to improve renal salvage. The wound and area around the injured kidney are packed with laparotomy pads to control bleeding with a planned return in 24 hours to explore and evaluate the extent of injury. This approach is commonly used by trauma surgeons in patients with extensive injuries and has long been used by general surgeons. It may well be useful in managing complex renal injuries to avoid total nephrectomy. The ability to reconstruct an injured kidney depends on numerous factors. The unstable patient, with low body temperature and poor coagulation, cannot risk an attempt at renal repair if a normal contralateral kidney is present (Davis et al, 2006). As noted earlier, damage control (packing the wound to control bleeding and attempting to correct metabolic and coagulation abnormalities) with a plan to return for corrective surgery within 24 hours provides an option in management. Total nephrectomy would be indicated immediately in extensive renal injuries when the patient’s life would be threatened by attempted renal repair. When Nash and colleagues (1995) examined the reasons for nephrectomy in patients with renal injuries, 77% required removal because of the extent of parenchymal, vascular, or combined injury and the remaining 23% required nephrectomy in otherwise reconstructable kidneys because of hemodynamic instability. Persistent urinary extravasation can result in urinoma, perinephric infection, and even renal loss. These patients are initially administered systemic antibiotics and carefully observed with appropriate antibiotics. In a high percentage, the extravasation resolves spontaneously (Matthews et al, 1997). Should it persist, placement of an internal ureteral stent often corrects the problem. A nonoperative approach with careful observation usually results in a functional renal unit.
Renal Injuries
Presentation
Hematuria
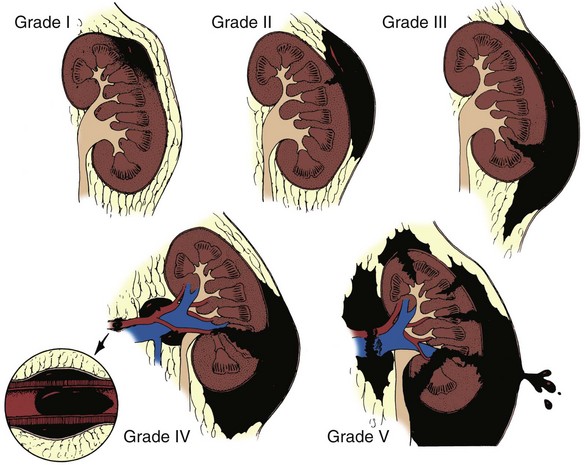
Classification
GRADE*
TYPE
DESCRIPTION
I
Contusion
Microscopic or gross hematuria, urologic studies normal
Hematoma
Subcapsular, nonexpanding without parenchymal laceration
II
Hematoma
Nonexpanding perirenal hematoma confined to renal retroperitoneum
Laceration
<1 cm parenchymal depth of renal cortex without urinary extravasation
III
Laceration
>1 cm parenchymal depth of renal cortex without collecting system rupture or urinary extravasation
IV
Laceration
Parenchymal laceration extending through renal cortex, medulla, and collecting system
Vascular
Main renal artery or vein injury with contained hemorrhage
V
Laceration
Completely shattered kidney
Vascular
Avulsion of renal hilum, devascularizing the kidney
Staging
Indications for Renal Imaging
Imaging Studies
Nonoperative Management
Isolated Renal Injuries
Operative Management
Renal Exploration
Is Early Vessel Isolation Necessary?
Renal Reconstruction
Renovascular Injuries
Damage Control
Indications for Nephrectomy
Complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Upper Urinary Tract Trauma