Curtis A. Pettaway, MD, Raymond S. Lance, MD, John W. Davis, MD A detailed discussion of surgical management of penile cancer is undertaken in Chapter 35. Here we review the epidemiology, etiology, and natural history of squamous carcinoma and its contemporary management. Reports have confirmed the importance of pathologic stage and histologic features of the primary tumor as well as the presence and extent of lymph node metastasis in determining prognosis and treatment planning of penile squamous carcinoma (Ravi, 1993b; McDougal, 1995; Theodorescu et al, 1996; Pizzocaro et al, 1997; Slaton et al, 2001). In addition, developments in staging of the disease, including novel imaging modalities and the use of lymphatic mapping, and modified surgical approaches to improve staging accuracy and reduce potential morbidities are presented. The selection of patients for organ-preserving surgical strategies is discussed. Please see the Expert Consult website Benign lesions of the penis include those of both a cystic and solid nature. Cystic lesions of shaft skin include congenital and acquired inclusion cysts, retention cysts, syringomas, and neurilemomas. Congenital inclusion cysts have occurred in the penoscrotal raphe (Cole and Helwig, 1976). Acquired inclusion cysts from circumcision or trauma are more common. Retention cysts arise from the sebaceous glands located on the mucosal surface of the prepuce and on the skin of the penile shaft. Retention cysts may arise in the parameatal area as a result of obstruction of the urethral glands (Shiraki, 1975). Syringomas, which are benign tumors of the sweat glands, may become large and symptomatic (Lipshutz et al, 1991; Sola Casas et al, 1993). Neurilemomas have been reported in the frenulum and prepuce (Chan et al, 1990; Hamilton et al, 1996). Benign tumors of the supporting structures include angiomas, fibromas, neuromas, lipomas, and myomas. Angiomas are usually superficial and appear most frequently as punctate reddish papules or macules on the corona. They resemble the small angiokeratomas found on the scrotum. Neuromas present as firm, whitish papules at the corona or frenulum (Montgomery et al, 1990). Fibroepithelial polyps may occasionally arise from the glans (Yildrim et al, 2004). Penile masses and deformities, or pseudotumors, may develop after self-administered injections or implantation of foreign bodies (Nitidandhaprabhas, 1975). Testosterone in oil (Zalar et al, 1969) and other common oils (Engelman et al, 1974) have been applied to or injected into the penis, producing a destructive lipogranulomatous process that may grossly mimic carcinoma. Pyogenic granuloma may arise at the site of self-injection in impotence therapy (Summers, 1990). Early or atypical Peyronie plaques may present as masses within the shaft and base of the penis. On occasion, phlebitis, lymphangitis, and angiitis may produce subcutaneous cords or nodules in the penis (Grossman et al, 1965; Ball and Pickett, 1975). Pearly penile papules, hirsute papillomas, and coronal papillae are normal and commonly encountered lesions of the glans penis (Fig. 34–1A; see also Fig. 15–42C). They occur in about 15% of postpubertal men and are more common in uncircumcised men (Neinstein and Goldenring, 1984). These lesions present as linear, curved, or irregular rows of conical or globular excrescences, varying from white to yellow to red, arranged along the coronal sulcus. They are considered acral angiofibromas. When larger than usual they may be mistaken for condylomata acuminata (Evans and Patten, 1990). Treatment is usually unnecessary, but when it is indicated success has been reported with fulguration with the carbon dioxide laser (Magid and Garden, 1989; Lane et al, 2002). These lesions have not been associated with malignant transformation (Tannenbaum and Becker, 1965; Ferenczy et al, 1990). Zoon balanitis is characterized by a shiny, erythematous plaque or erosion that on biopsy demonstrates normal cell layers but a dense plasma cell infiltrate (see Fig. 15–42D). It most commonly involves the glans but may involve the prepuce. Circumcision is the time tested treatment of this condition, although there are reports of successful management with 1% pimecrolimus cream (Bardazzi, et al, 2008). Carbon dioxide laser ablation has also been reported to be successful in treating Zoon balanitis (Baldwin and Geronemus, 1989). The full range of rashes and ulceration due to irritation, allergy, or infection must be considered in the differential diagnosis of the cutaneous lesion (Schneede et al, 2003). Some histologically benign penile lesions have been recognized as having malignant potential or close association with the development of squamous carcinoma. In one large series, 42% of patients with squamous cell cancer had a history of preexisting penile lesions (Bouchot et al, 1989). Although the incidence of progression of these lesions to squamous cell carcinoma is not known, all have been associated with the disease. The penile cutaneous horn is a rare lesion. It usually develops over a preexisting skin lesion (wart, nevus, traumatic abrasion, or malignant neoplasm) and is characterized by overgrowth and cornification of the epithelium, which forms a solid protuberance. On microscopic examination, extreme hyperkeratosis, dyskeratosis, and acanthosis are noted. It is associated with human papillomavirus (HPV) type 16. Treatment consists of surgical excision with a margin of normal tissue about the base of the horn. These lesions may recur and may demonstrate malignant change on subsequent biopsy, even when initial histologic appearance is benign (Fields et al, 1987). Because this tumor may evolve into a carcinoma or may develop as a result of an underlying carcinoma, careful histologic evaluation of the base and close follow-up of the excision site are essential (see Fig. 34–1A) (Pressman et al, 1962; Hassan et al, 1967; Solivan et al, 1990). These unusual lesions present as hyperkeratotic, micaceous growths on the glans and may have some of the microscopic features of verrucous carcinoma (see Fig. 15–39). They tend to recur and may represent an early form of that tumor (Jenkins and Jakubovic, 1988; Gray and Ansell, 1990). Treatment includes excision, laser ablation, or cryotherapy. These lesions require aggressive treatment and close follow-up. Fibrosarcoma of the glans after treatment of a pseudoepitheliomatous micaceous and keratotic balanitis lesion with cryotherapy has been reported (Irvine et al, 1987). This genital variation of lichen sclerosus atrophicus presents as a whitish patch on the prepuce or glans, often involving the meatus and sometimes extending into the fossa navicularis (see Fig. 15–12). The lesions may be multiple and may assume a mosaic appearance. The meatus may appear white, indurated, and edematous. Glanular erosions, fissures, and meatal stenosis may occur. The disorder is most common in uncircumcised men and occurs most commonly in middle-aged men, but it does occur in boys (McKay et al, 1975). Symptoms include pain, dyspareunia, pruritus, painful erections, and urinary obstruction (Bainbridge et al, 1971). On histologic examination these lesions show an atrophic epidermis with loss of the rete pegs and homogenization of collagen on the upper third of the dermis combined with a zone of lymphocytic and histiocytic infiltration. They resemble the lesions of lichen sclerosus et atrophicus found elsewhere (Laymon and Freeman, 1944). There are reports documenting the association of male lichen sclerosis (LS) with squamous cell carcinoma as well as with the development of carcinoma long after a lesion of lichen sclerosis has been treated (Laymon and Freeman, 1944; Bart and Kopf, 1978; Jamieson et al, 1986; Dore et al, 1990; Simonart et al, 1998; Velazquez and Cubilla, 2003; Kumaran and Kanwar, 2004). Cubilla and coworkers (2004) describe a well-differentiated nonverruciform variant of squamous cell carcinoma associated with lichen sclerosus et atrophicus that preferentially involves the prepuce. The etiology of male lichen sclerosis is unknown; however, a recent study identified Borrelia burgdorferi infection in affected tissues in the early course of the disease. Floating microscopy was used to examine 60 cases of lichen sclerosis, and B. burgdorferi species were identified in 63% of the lichen sclerosis tissues and none of the controls (Eisendle et al, 2008). Whether this organism is causative is unproven at this time and requires additional study. Treatment involves clobetasol propionate cream for 2 to 3 months (Pugliese et al, 2007). Meatal stenosis is a common problem often requiring repeated dilations, corticosteroid injection, or even formal reconstructive surgery (Poynter and Levy, 1967). Close follow-up is essential, with biopsy if a change in appearance or behavior occurs. Key Points Benign and Premalignant Cutaneous Lesions There is increasing evidence to suggest that a number of penile lesions share viral etiologies. Condyloma acuminatum and bowenoid papulosis appear to be related to infection with human papillomavirus. Human herpesvirus 8, also known as Kaposi sarcoma–associated herpesvirus, is strongly suspected of being the etiologic agent of epidemic (AIDS-related) Kaposi sarcoma (Miller et al, 1996; Simpson et al, 1996; Jaffe and Pellett, 1999; Dianzani et al, 2004). Condylomata acuminata are soft, papillomatous growths generally considered to be benign (see Fig. 15–20G). Also known as genital warts or venereal warts, they have a predilection for the moist, glabrous areas of the body and the mucocutaneous surfaces of the perineal and genital areas. The lesions are soft and friable and may occur singly on a pedicle or in a moruloid cluster on a broad base. These lesions are rare before puberty (Redman and Meacham, 1973; Copulsky et al, 1975) and when encountered may suggest sexual abuse (Handly et al, 1993). In the male, condylomata occur most commonly on the glans, the penile shaft, and the prepuce. The meatus should also be carefully inspected. Lesions recur frequently, both in new and in previously treated sites. Approximately 5% of patients will demonstrate urethral involvement, which may extend to the prostatic urethra (Culp et al, 1944). Rarely, extreme involvement of the urethra may require urethroplasty (Feneley et al, 1992). Bladder involvement, although rare, is extremely difficult to treat effectively (Bissada et al, 1974). On microscopic examination, condylomata acuminata demonstrate an outer layer of keratinized tissue covering papillary fronds, which are supported by connective tissue stroma. The epithelial layer consists of well-ordered rows of squamous cells. A dermal lymphocytic infiltrate is usually present. Treatment of these lesions with podophyllin may induce histologic changes suggestive of carcinoma (King and Sullivan, 1947). Consequently, preliminary biopsy of large lesions that appear to be condylomata acuminata should precede any treatment with topical podophyllin. Interest in genital condylomata has increased dramatically, stimulated by an increased understanding of the relationship between HPV infection and certain human cancers. The terms genital condyloma, venereal warts, genital warts, and genital HPV infection all refer to a sexually transmitted disease caused by HPV. Although HPV is not a reportable sexually transmitted disease, a current estimate puts the number of new infections at 500,000 to 1 million annually (Stone, 1989). A study of 463 men aged 18 to 40 years analyzed samples from the glans/corona, penile shaft, scrotum, urethra, perianal area, anal canal, and semen and detected HPV in 51.2% (Nielson et al, 2007). Giuliano and colleagues (2009) found the prevalence of HPV in U.S. males to be 51.7%, in Brazilian men to be 69.4%, and in Mexican men to be 56.1%. In a study of 379 adult heterosexual males, HPV infection was localized to the penile shaft (52%), scrotum (40%), glans/corona (32%), urine (10%), and semen (6%) (Hernandez et al, 2008). In a cohort of 379 adult heterosexual males, the overall incidence of HPV infection was 52%, with 60% of the uncircumcised and 50% of the circumcised males found to have HPV infection. There was a higher prevalence of oncogenic HPV in the uncircumcised group (Hernandez et al, 2008). Factors associated with higher rates of infection with HPV include presence of foreskin, increasing numbers of sexual partners, lack of condom use, and smoking (Giuliano et al, 2009). The overall prevalence of HPV in females was found to be 26.8% among U.S. females aged 14 to 59 years and highest among women aged 20 to 24 years (44.8%) (Dunne et al, 2007). HPV is recognized as the principal etiologic agent in cervical dysplasia and cervical cancer (Lancaster et al, 1986; Alani and Munger, 1998; Gross and Pfister, 2004). Significant numbers of male partners of women with cervical condylomata will have lesions not identified by simple inspection and may not be aware that they are infected or have the potential to infect others (Sedlacek et al, 1986). On histologic examination, the koilocyte—a cell characterized by an empty cavity surrounding an atypical nucleus—is pathognomonic for HPV infection (Schneider, 1989). DNA hybridization techniques have been used to identify and classify HPV infections, and some 60 genotypes of HPV virus have been identified that involve the genital tract (Nielson et al, 2007). Virus types 6, 11, and 42 to 44 are associated with gross condylomata and low-grade dysplasia. Types 16, 18, 31, 33, 35, and 39 have a higher association with malignant disease (Smotkin, 1989). More recently, reports have suggested that tumor virus transforming proteins from HPV types 16 and 18, particularly the E6 and E7 proteins, may target tumor suppressor gene products pRB and TP53 and may be the causative agents in a subset of penile cancers (Levi et al, 1998; Griffiths and Mellon, 1999). E6 appears to bind to cellular tumor suppressor protein TP53, leading to its rapid degradation, resulting in chromosome instability, DNA mutations, and aneuploidy. E7 binds to and phosphorylates the pRB retinoblastoma protein, leading to the release of transcription factor E2F that activates mitosis (zur Hausen, 1996). Human immunodeficiency virus (HIV) infection may predispose affected patients to rapid development of squamous carcinoma from preexisting condyloma infection (Sanders, 1997). Subclinical disease may be detected by the application of 5% acetic acid solution to the penis, followed by inspection with a magnifying glass. Lesions will turn white, and flat lesions often invisible on regular inspection may be detected. These aceto-white lesions are not always due to HPV, and biopsy must be performed to confirm the diagnosis (Krebs, 1987). Careful inspection of the base of the shaft, the scrotum, and the inguinal folds is essential. The meatus should be examined; if lesions are present, urethroscopy should be performed (Culp et al, 1944; Barrasso et al, 1987). A variety of treatments for genital warts are available but none has been proven to reduce transmission to sexual partners nor to prevent progression to dysplasia or cancer. In addition, recurrence after treatment is quite common. The more commonly utilized treatments include (1) podophyllotoxin 0.5% solution or gel, (2) trichloroacetic acid 35% to 85%, (3) cryotherapy with liquid nitrogen, (4) electrofulguration, (5) CO2 laser therapy, and (6) imiquimod 5% cream (Dupin, 2004; Buechner, 2002). Surgical laser therapy has been used extensively in the management of condylomata and is covered in Chapter 35. Surgical therapy with use of a pediatric resectoscope may be helpful in debulking large intraurethral lesions. The lowest power required to resect the lesions should be used, and electrocautery should be minimized to avoid the development of urethral stricture. Whether laser therapy, electrocautery, or cryotherapy, significant rates of recurrence have been noted within the first 6 months after treatment (i.e., 40%-80%, Dupin, 2004). Topical podophyllin (Condylox 0.5%) or trichloroacetic acid has been a well-established and often successful treatment for small condyloma lesions (Culp et al, 1944; Kinghorn et al, 1993). More recently, however, imiquimod cream (5%) has become the topical treatment of choice for condyloma. Imiquimod is an immune modulator that enhances natural killer cell activity (Buechner, 2002; Sanchez-Ortiz and Pettaway, 2003). Importantly, immune modulators and antiviral agents have the potential to affect the viral load. Various interferons have been used in condyloma treatment (Geffen et al, 1984). A randomized study has shown that short-term intralesional interferon alfa-2b has activity against condyloma (Eron et al, 1986). The outcome of studies using other interferons has been less clear (Zouboulis et al, 1991). Interferon therapy continues to be reserved for extensive and recalcitrant lesions (Krebs, 1989a, 1989b; Ferenczy, 1990). Another recent antiviral agent shown to have activity against HPV includes (s)-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine (cidofovir), an acyclic nucleoside phosphonate with broad-spectrum antiviral activity against DNA viruses. A 1% gel preparation of cidofovir applied daily every other week for 6 cycles was shown to be superior to placebo in a double-blind placebo-controlled trial with a complete response of 47% in treated patients (Snoeck et al, 2001). Intraurethral lesions may be extremely difficult to treat. 5-Fluorouracil cream applied weekly for 3 weeks has been successful in eliminating urethral lesions (Bissada et al, 1974; Dretler and Klein, 1975; Boxer and Skinner, 1977). Care must be taken to work the cream down the urethra and to avoid exposure of the scrotal skin. Use of a scrotal support or zinc oxide cream may be helpful. The addition of 5-fluorouracil cream to laser therapy did not improve the success rate in one study (Carpiniello et al, 1987). HPV infection is common and, as noted earlier, potentially carcinogenic. Condylomata have been associated with squamous cell carcinoma of the penis (Beggs and Spratt, 1964; Dawson et al, 1965; Rhatigan et al, 1972). Malignant transformation of condyloma to squamous cell carcinoma has been reported (Boxer and Skinner, 1977; Coetzee, 1977; Malek et al, 1993). Condylomata acuminata located in the perianal, scrotal, and oral areas have also demonstrated malignant degeneration (Siegel, 1962; Burmer et al, 1993). An increased incidence of penile intraepithelial neoplasia has been found in the male partners of women with cervical intraepithelial neoplasia (Barrasso et al, 1987; Iversen et al, 1997). Thus preventive strategies are relevant. Recently, a quadrivalent vaccine (Gardasil, approved by the Food and Drug Administration, June 2006) that protects against HPV types 5, 11, 16, and 18 in women ages 9 to 26 years (Watson et al, 2008) has become available. Currently this vaccine is not approved for use in young men, although considerable debate suggests a future role for HPV vaccination in men, particularly among organ transplant recipients and homosexual men (Gillison et al, 2008). Cost considerations add to the controversy of including young men in HPV vaccination programs at $120.50 per dose (Hoffner, 2009). Bowenoid papulosis presents as multiple papules on the penile skin or female vulva, usually during the second or third decade of life. The lesions are usually pigmented and range from 0.2 to 3.0 cm in diameter, and smaller lesions may coalesce into larger ones (Patterson et al, 1986). Pigmented lesions present on the penile skin, whereas glanular lesions tend to be flat papules (Gross et al, 1985). Diagnosis is confirmed by biopsy (Peters and Perry, 1981). These lesions meet all the histologic criteria of carcinoma in situ but display differing growth patterns relative to flat, endophytic, or exophytic clinical appearance (Wade et al, 1978; Peters and Perry, 1981; Gross et al, 1985; Patterson et al, 1986; Bhojwani et al, 1997). DNA sequences suggestive of HPV-16 have been found in specimens of bowenoid papulosis, and a causative role for HPV is suspected (Gross et al, 1985; Endo et al, 2003). Whereas histologically this condition is a carcinoma in situ, the clinical course of bowenoid papulosis is invariably benign (Su and Shipley, 1997). Kaposi sarcoma, first described in 1972, is a tumor of the reticuloendothelial system (Kaposi, 1982). It presents as a cutaneous neovascular lesion, a raised, painful, bleeding papule or ulcer with bluish discoloration. On histologic examination the tumor is vasoformative with endothelial proliferation and spindle cell formation. The classic and immunosuppressive forms of the disease are considered nonepidemic. Nonepidemic Kaposi sarcoma limited to penile involvement should be aggressively treated because it is rarely associated with diffuse organ involvement. Localized surgical excision or small-field external beam or electron beam radiation has been effective (Lands et al, 1992). With wider areas of involvement, partial penectomy is indicated. In the immunosuppressed patient Kaposi sarcoma will often regress with the discontinuation of immunosuppressive therapy. If regression does not occur, local excision or radiation should be considered. Systemic management for multisystem involvement has employed interferon and cytotoxic therapy (National Cancer Institute Position Statement, 1990). In the patient with AIDS, the underlying immune deficiency predisposes the host to Kaposi sarcoma by a factor of 7000 (Miles, 1994). The first case of HIV-related epidemic Kaposi sarcoma was reported in 1981 (Friedman-Kien, 1981) and the first with penile involvement in 1986 (Seftel et al, 1986). Subsequently, Kaposi sarcoma of the penis has become a relatively common lesion in the patient with AIDS. Penile involvement is more common in the homosexual man than in others with AIDS. In the first 1000 cases of AIDS reported by the Centers for Disease Control and Prevention, incidence of penile Kaposi sarcoma was 44% in homosexual and bisexual patients compared with only 16% in intravenous drug abusers with AIDS and 0% of hemophiliac patients with AIDS (Jaffe et al, 1983; Bayne and Wise, 1988). Several reports suggest a strong relationship between infection with human herpesvirus 8, also known as Kaposi sarcoma–related herpesvirus, and the development of Kaposi sarcoma lesions in patients with HIV infection (Jaffe and Pellett, 1999; Sitas et al, 1999). Largely because of the explosion of AIDS in the past decade, Kaposi sarcoma of the penis is now probably the second most common malignant neoplasm of the penis after squamous cell carcinoma. Some studies have found epidemic Kaposi sarcoma in patients who are HIV negative, which suggests that certain sexual practices and a separate sexually transmitted agent may be responsible for this form of the disease (Miles, 1994; Chitale et al, 2002). Recently human herpesvirus 8 has been isolated from penile Kaposi lesions in patients who were HIV negative supporting the notion that this may be sexually transmitted potentially via homosexual practices (Morelli et al, 2003). Whereas Kaposi sarcoma may be the presenting sign of the disease in many patients with AIDS, early involvement of the penis is rare in this group (Grunwald et al, 1994). Treatment is directed toward palliation (Lowe et al, 1989). Glans penis or corpus spongiosum involvement may produce urethral obstruction, necessitating proximal urethrostomy. This will usually allow voiding in the upright position. With large lesions involving the penis, partial or total penectomy may be necessary. Radiation therapy and use of the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser to alleviate distal urethral obstruction have also been reported (Wishnow and Johnson, 1988; Ruszczak et al, 1996). The Buschke-Löwenstein tumor was initially described by Buschke and Löwenstein in 1925 and later by Löwenstein in 1939 in the United States. Ackerman (1948) described a histologically similar tumor presenting in the oral cavity. Verrucous carcinomas of the larynx, vulva, and penis were described by Goethals and colleagues (1963) (see Fig. 34–1B). The true incidence of the Buschke-Löwenstein tumor is unknown, but it is probably higher than reported because many cases have been labeled low-grade squamous carcinoma of the penis. Retrospective analyses of several reports have revealed a number of cases of verrucous cancer or giant condylomata under the category of low-grade squamous cell carcinomas (Davies, 1965; Hanash et al, 1970; Grussendorf-Conen, 1997). Key Points Virus-Related Dermatologic Lesions The Buschke-Löwenstein tumor differs from condyloma acuminatum in that condylomata, regardless of size, always remain superficial and never invade adjacent tissue. Buschke-Löwenstein tumor displaces, invades, and destroys adjacent structures by compression. Aside from this unrestrained local growth, it demonstrates no signs of malignant change on histologic examination and does not metastasize. On microscopic examination, the tumor forms a luxuriant mass composed of broad rounded rete pegs, often extending far into underlying tissue. The pegs are composed of well-differentiated squamous cells that show no cellular anaplasia. These epithelial pegs are characteristically surrounded by a dense band of acute and chronic inflammatory cells. As with condyloma acuminatum, the etiology may be viral (Dawson et al, 1965; Ubben et al, 1979; Antony et al, 2003). DNA from HPV types 6 and 11 has been identified in these tumors (Boshart and zur Hausen, 1986). Lymph node metastases are rare with verrucous carcinoma (Ackerman, 1948; Davies, 1965; Seixas et al, 1994), and their presence probably reflects malignant degeneration in the primary lesion. Such changes are known to occur in verrucous carcinoma of nonpenile sites (Davies, 1965; Dawson et al, 1965). Anecdotal cases of malignant degeneration in association with penile carcinoma have been reported (Youngberg et al, 1983). Either excisional biopsy or multiple deep biopsies are required to distinguish the lesion from true penile carcinoma. Treatment consists of excision, sparing as much of the penis as possible. Large lesions may necessitate total penectomy. Recurrence is common, and close follow-up is essential. Topical therapy with either podophyllin or 5-fluorouracil has been unsuccessful, probably because the characteristic thickened stratum corneum is impervious to the medication (Bruns et al, 1975). Radiation therapy is ineffective and has been associated with subsequent rapid malignant changes of verrucous carcinomas in the penis and other locations (Lepor and Leffler, 1960; Kraus and Perez-Mesa, 1966; Proffitt et al, 1970; Fukunaga et al, 1994). Bleomycin has been used in both a primary and an adjunctive mode for verrucous carcinoma (Mishima and Matunaka, 1972). Successful treatment of a Buschke-Löwenstein tumor with systemic interferon therapy combined with Nd:YAG laser therapy has been reported (Gilbert and Beckert, 1990). Cryosurgery has also been employed with success (Michelman et al, 2002). The erythroplasia originally described by Queyrat in 1911 consists of a red, velvety, well-marginated lesion of the glans penis or, less frequently, the prepuce of the uncircumcised man (Aragona et al, 1985). It may ulcerate and may be associated with discharge and pain. On histologic examination the normal mucosa is replaced by atypical hyperplastic cells characterized by disorientation, vacuolation, multiple hyperchromatic nuclei, and mitotic figures at all levels. The epithelial rete extends into the submucosa and appears elongated, broadened, and bulbous. The submucosa shows capillary proliferation and ectasia with a surrounding inflammatory infiltrate that is usually rich in plasma cells. These microscopic features distinguish erythroplasia of Queyrat from chronic localized balanitis. Human papillomavirus (HPV) has been identified in penile carcinoma in situ (Pfister and Haneke, 1984). Progression to invasive carcinoma can occur in 10% to 33% of cases (Buechner, 2002; Bleeker et al, 2009). In 1912 Bowen described an intraepithelial neoplasm of the skin associated with a high occurrence of subsequent internal malignant disease as a distinct entity. Bowen disease and erythroplasia of Queyrat are histologically similar (Graham and Helwig, 1973) (see Fig. 34–1C on the Expert Consult website Treatment is based on proper histopathologic confirmation of malignancy with multiple biopsies of adequate depth to rule out invasion. When lesions are located on the foreskin, circumcision or excision with a 5-mm margin is adequate for local control (Bissada, 1992). In this regard, lesions on the glans penis are difficult to treat by excisional strategies while maintaining normal penile anatomy. Alternative strategies include topical 5-fluorouracil cream (Lewis and Bendl, 1971; Graham and Helwig, 1973; Goette, 1974), 5% imiquimod cream (Danielson et al, 2003), and ablation with the neodymium:yttrium-aluminum-garnet (Nd:YAG) (Landthaler et al, 1986; Frimberger et al, 2002a), potassium titanyl phosphate (KTP) 532-nm, or carbon dioxide lasers (Rosemberg and Fuller, 1980; Tietjen and Malek, 1998; van Bezooijen et al, 2001). Such strategies have been shown to produce excellent cosmetic and functional results. Radiation therapy can be used to treat tumors that are resistant to topical treatment, especially among patients who are not surgical candidates (Kelley et al, 1974; Grabstald and Kelley, 1980). Penile carcinoma accounts for 0.4% to 0.6% of all malignant neoplasms among men in the United States and Europe; it may represent up to 10% of malignant neoplasms in men in some Asian, African, and South American countries (Gloeckler-Ries et al, 1990; Vatanasapt et al, 1995). However, reports suggest that the incidence of penile cancer is decreasing in many countries, including Finland, the United States, India, and other Asian countries (Maiche et al, 1992; Frisch et al, 1995; Vatanasapt et al, 1995; Yeole and Jussawalla, 1997). The reasons are unclear but may be related in part to increased attention to personal hygiene. Penile cancer is a disease of older men, with an abrupt increase in incidence in the sixth decade of life (Persky, 1977). In two studies the mean age was 58 years (Gursel et al, 1973) and 55 years (Derrick et al, 1973). The tumor is not unusual in younger men; in one large series, 22% of patients were younger than 40 years and 7% were younger than 30 years (Dean, 1935); the disease has also been reported in children (Kini, 1944; Narasimharao et al, 1985). The Surveillance, Epidemiology and End Results (SEER) database reveals no racial difference in incidence of penile cancer between black and white men in the United States (incidence for white men, 0.8 of 100,000; for black men, 0.7 of 100,000) (Vatanasapt et al, 1995). However, a study using SEER data suggested that race is associated with outcome. Rippentrop and colleagues (2004) noted there were 1605 patients diagnosed with penile cancer from 1973 to 1998, with 22.4% (360) dying of the disease. They found factors independently predictive of worsened survival to be higher stage at diagnosis, age older than 65 years, African-American ethnicity, and disease within lymph nodes. These researchers demonstrated a statistically significant disease-specific risk of death that was 2.2-fold higher in African-American patients than in white patients. Although the reason for this disparity is likely to be multifactorial, possibilities include differences in cancer biology, in health care access, or in treatment. These provocative findings clearly deserve further study. The incidence of carcinoma of the penis varies according to circumcision practice, hygienic standard, phimosis, number of sexual partners, HPV infection, exposure to tobacco products, and other factors (Barrasso et al, 1987; Maiche, 1992; Maden et al, 1993; Misra et al, 2004). Neonatal circumcision has been well established as a prophylactic measure that virtually eliminates the occurrence of penile carcinoma because it eliminates the closed preputial environment where penile carcinoma develops. The chronic irritative effects of smegma, a byproduct of bacterial action on desquamated cells that are within the preputial sac, have been proposed as an etiologic agent. Although definitive evidence that human smegma itself is a carcinogen has not been established (Reddy and Baruah, 1963), its relationship to the development of penile carcinoma has been widely observed. Improper hygiene can lead to buildup of smegma beneath the preputial foreskin, with resulting inflammation. Healing by fibrosis leads to phimosis of the preputial skin, which tends to perpetuate the cycle. Phimosis is found in 25% to 75% of patients described in most large series. Reddy and associates (1984) studied the foreskins of 26 men undergoing circumcision because of phimosis and found epithelial atypia in one third of the specimens. Carcinoma of the penis is rare among the Jewish population, for whom neonatal circumcision is a universal practice (Licklider, 1961). Similarly, in the United States, where neonatal circumcision is widely practiced, penile cancer represents less than 1% of male malignant neoplasms. Among uncircumcised tribes of Africa and within uncircumcised Asian cultures, penile cancer may amount to 10% to 20% of all male malignant neoplasms (Dodge, 1965; Narayana et al, 1982). Data from most large series show that penile cancer is rare among neonatally circumcised individuals but more frequent when circumcision is delayed until puberty (Frew et al, 1967; Gursel et al, 1973; Johnson et al, 1973). Adult circumcision appears to offer little or no protection from subsequent development of the disease (Maden et al, 1993). These data suggest that the critical period of exposure to certain etiologic agents may have already occurred at puberty and certainly by adulthood, rendering later circumcision relatively ineffective as a prophylactic tool for penile cancer. Recent population-based data reveal that although neonatal circumcision is highly protective for invasive penile cancer it does not afford the same level of protection for carcinoma in situ. Schoen and colleagues (2000) evaluated the incidence of invasive penile cancer or carcinoma in situ during a 10-year period and found only 2 cases of 89 (2.3%) occurring among neonatally circumcised men, whereas of 118 men with carcinoma in situ, 16 cases were noted in men circumcised at birth (15.7%). Considering that the protective effects of circumcision on invasive penile cancer are likely to be mediated by avoidance of phimosis, it is noteworthy that another study associated phimosis with the development of invasive penile cancer but not carcinoma in situ (Hung-fu et al, 2001). Male circumcision has also been shown to be effective against HIV-1 infection. This effect was shown to be specific by Reynolds and colleagues (2004). There was no protective effect of circumcision for other sexually transmitted diseases, such as herpes simplex virus type 2 infection, syphilis, or gonorrhea. HPV infection and exposure to tobacco products appear to be associated with development of penile cancer. Epidemiologic data provided the first clues to a relationship between a sexually transmitted agent and cancer by demonstrating that the wives or ex-wives of men with penile cancer had a threefold higher risk of cervical carcinoma (Graham et al, 1979). Further investigation revealed that the male partners of women with cervical intraepithelial neoplasia had a significantly higher incidence of penile intraepithelial neoplasia (Barrasso et al, 1987). These same male patients were also found to have a greater incidence of HPV infection. Advanced molecular biologic techniques such as polymerase chain reaction and in-situ hybridization have provided increased evidence for an etiologic role of HPV by identifying specific DNA sequences from different HPV types in primary penile lesions (malignant and benign) but not in normal foreskins (Varma et al, 1991; Iwasawa et al, 1993). More than 25 types of HPV infect genital sites. HPV types 6 and 11 are most commonly associated with nondysplastic lesions such as genital warts, but these are also noted in nonmetastatic verrucous carcinomas. In contrast, HPV types 16, 18, 31, and 33 are associated with in-situ and invasive carcinomas (Wiener and Walther, 1995). HPV-16 appears to be the most frequently detected type in primary carcinomas and has also been detected in metastatic lesions (Varma et al, 1991; Iwasawa et al, 1993; Wiener and Walther, 1995). As noted previously, the HPV genome encodes oncoprotein E6, which complexes with the tumor suppressor protein TP53, and oncoprotein E7, which binds the retinoblastoma (RB) protein, thus affecting cell cycle regulation (Munger et al, 1989; zur Hausen, 1996; Levi et al, 1998; Griffiths and Mellon, 1999) via the P14ARF/MDM2/p53 and p15INK4a/cyclin D/Rb pathways (Bleeker et al, 2009). Maden and colleagues (1993) found that the incidence of HPV infection directly correlated with the number of lifetime sexual partners, which was also related to risk of penile cancer. Furthermore, Castellsague and colleagues (1997) noted a direct correlation between the number of sexual partners, HPV-infected men, and incidence of cervical neoplasia among their female partners. Thus, for both cervical and penile cancer, HPV infection represents a preventable etiology. Poblet and coworkers (1999) reported on two cases of coexisting HIV-1 and HPV infection and postulated that HIV-1 could synergize with HPV to increase the progression of HPV penile lesions into penile carcinoma. Although there is evidence supporting this effect in cervical and anal neoplasia, definitive proof for penile cancer awaits further study (Northfelt, 1994). Although HPV infection is probably an important factor in the development of penile cancer, its presence is not invariable (31% to 63% of patients with penile carcinoma test positive) (Wiener and Walther, 1995), indicating that additional factors may be involved in the development of the disease or its subtypes. Additional evidence for Rubin and associates (2001), who performed a sensitive polymerase chain reaction assay, provided this hypothesis-based essay on penile cancer specimens from the United States and Paraguay. Overall, 42% of penile carcinomas were HPV positive. However, only 34.9% and 33.3% of keratinizing and verrucous carcinomas, respectively, were positive, whereas 80% and 100% of basaloid and warty tumor subtypes, respectively, exhibited HPV DNA. Other non–HPV-dependent molecular events leading to penile carcinogenesis have been described, including silencing of the CDK2NA locus via promoter hypermethylation, the expression of genes that target the INK4a/ARF locus, other gene mutations affecting TP53, and P14ARF as well as MDM2 overexpression (reviewed in Ferreux et al, 2003; Bleeker et al, 2009). Four studies have shown a significant association between exposure to cigarette smoke and development of penile cancer (Hellberg et al, 1987; Daling et al, 1992; Maden et al, 1993; Harish and Ravi, 1995). Hellberg and colleagues (1987) studied the smoking history of 244 men with penile cancer and matched controls. They found a significantly increased odds ratio for penile cancer based on whether an individual had smoked, and the risk increased with the number of cigarettes smoked. This observation held even when the presence of phimosis was controlled. Harish and Ravi (1995) extended these observations by showing that all forms of tobacco products, including cigarettes, chewing tobacco, and snuff, were significantly and independently related to the incidence of penile cancer subsequent to multivariate regression analysis. It has been hypothesized that tobacco products can act in the presence of HPV infection or bacteria associated with chronic inflammation to promote malignant transformation. These same risk factors are also common to other anogenital carcinomas (Daling et al, 1992; Maden et al, 1993). Penile trauma may be another risk factor for penile cancer. The development of carcinoma in the scarred penile shaft after mutilating circumcision has been reported as a distinct entity (Bissada et al, 1986). Furthermore, Maden and colleagues (1993) found a greater than threefold risk of penile cancer in men with penile tears and rashes. A case-control study also revealed an odds ratio of 18:1 for the development of penile cancer for those men reporting a penile injury 2 years before the onset of the disease (Hung-fu et al, 2001). Genital ultraviolet radiation, alone and combined with 8-methoxypsoralen, increases the risk of squamous carcinoma at genital sites. A 12-year follow-up study reported that the risk of penile and scrotal cancer was increased 286 times that of the general population for those exposed to ultraviolet A photochemotherapy and 8-methoxysoralen (PUVA) (Stern et al, 1990). The risk was dose related. For those treated with ultraviolet B exposure, the risk was 4.6-fold enhanced. Another long-term follow-up study of PUVA-associated malignant neoplasia from Sweden revealed a 30-fold increased risk for skin cancer (but not for penile cancer) among males. In this study, PUVA was also associated with respiratory and pancreatic cancers (Lindelof et al, 1991). Lichen sclerosus (also known as balanitis xerotica obliterans) is a risk factor for the development of penile cancer. Studies have shown the incidence of subsequent cancer with long-term follow-up to be between 3% and 9% of men with lichen sclerosus (Depasquale et al, 2000; Micali et al, 2001). Velazquez and Cubilla (2003) studied lichen sclerosus occurring in association with penile cancer and noted its presence distinctly among the subset of penile carcinomas that were not associated with HPV. Larger studies performed in areas where the disease is endemic, incorporating the many risk factors for penile cancer into a multivariate analysis, are clearly needed to define which factors independently confer risk. Thus far, no convincing evidence has been found linking penile cancer to other factors such as occupation, other venereal diseases (gonorrhea, syphilis, and herpes), marijuana use, or alcohol intake (Maden et al, 1993). The role of routine neonatal circumcision as a preventive strategy for penile cancer has been, to say the least, a controversial topic. The position of the American Academy of Pediatrics has changed from one of denial of any medical benefits (Schoen et al, 1989) to the more moderate recent position stating, “There are potential medical benefits of newborn circumcision; however, these data are not sufficient to recommend routine neonatal circumcision. In such situations where the procedure is not essential to the child’s well being, parents should determine what is in the best interest of the child subsequent to being presented unbiased information” (Shapiro, 1999). Any argument against circumcision must consider that penile carcinoma represents the only neoplasm for which there exists a predictable and simple means of prophylaxis to spare the organ at risk (Dagher et al, 1973). Although circumcision can obviate the disease, especially where facilities for daily hygiene may be lacking, it may not be as important in countries where good hygiene is practiced. Frisch and colleagues (1995) reported a falling incidence of penile cancer (from 1.15 of 100,000 men to 0.82 of 100,000 men) in the Danish population, which has a circumcision rate of only 1.6%. They attributed this trend to improved hygiene because the incidence of dwellings having a bath facility increased from 35% in the 1940s to 90% in the 1990s. Thus, considering the benefits of circumcision (including the prevention of infections, HIV infection and its transmission, and penile and cervical cancer), enhanced education about the potential benefits of circumcision, especially in developing countries, seems rational (Schoen et al, 1989; Reynolds et al, 2004; Kinkade et al, 2005). Although neonatal circumcision and good hygiene to prevent the occurrence of phimosis represent important prevention strategies, additional efforts to prevent malignant transformation include avoidance of HPV infection potentially through condom use, of ultraviolet light exposure, and of tobacco products. Thus, modifiable behaviors can potentially prevent penile cancer (Munger et al, 1989; Maden et al, 1993; Harish and Ravi, 1995; Levi et al, 1998; Griffiths and Mellon, 1999; Bleeker et al, 2009). HPV vaccination could play an emerging role in the future with respect to preventing transmission of HPV between males and females and potentially penile cancer. To date two prophylactic HPV vaccines are available (HPV 16/18 vaccine Cervarix [GlaxoSmithKline] and the quadrivalent HPV 16/18/6/11 vaccine Gardasil [Sanofi Pasteur, Merck Sharpe & Dome]. The efficacy of preventing HPV infection among HPV negative women has been demonstrated (Harper et al, 2004; Villa et al, 2005). To date the safety and immunogenicity of the vaccines has also been established among adolescent boys. However, efficacy in prevention of HPV infection in such populations awaits the results of ongoing studies (Block et al, 2006; Bleeker et al, 2009). Key Points Epidemiology, Etiology, and Prevention Carcinoma of the penis usually begins with a small lesion that gradually extends to involve the entire glans, shaft, and corpora. The lesion may be papillary and exophytic or flat and ulcerative; if it is untreated, penile autoamputation may occur as a late result. The rates of growth of the papillary and ulcerative lesions are similar, but the flat, ulcerative tumor has a tendency toward earlier nodal metastasis and is associated with poorer 5-year survival rates (Dean, 1935; Marcial et al, 1962; Ornellas et al, 1994). Lesions larger than 5 cm (Beggs and Spratt, 1964) and those extending over 75% of the shaft (Staubitz et al, 1955) are also associated with an increased incidence of metastases and a decreased survival rate. However, others have not found a consistent relationship between lesion sizes, presence of metastases, and decreased survival (Ekstrom and Edsmyr, 1958; Puras et al, 1978). Buck fascia acts as a temporary natural barrier to local extension of the tumor, protecting the corporeal bodies from invasion. Penetration of Buck fascia and the tunica albuginea permits invasion of the vascular corpora and establishes the potential for vascular dissemination. Urethral and bladder involvement are rare (Riveros and Gorostiaga, 1962; Thomas and Small, 1968). The earliest route of dissemination from penile carcinoma is metastasis to the regional femoral and iliac nodes. A detailed description of lymphatic drainage of the penis is found elsewhere in this text and is well documented in the literature (Dewire and Lepor, 1992). Briefly, the lymphatics of the prepuce form a connecting network that joins with the lymphatics from the skin of the shaft. These tributaries drain into the superficial inguinal nodes (the nodes external to the fascia lata). The lymphatics of the glans join the lymphatics draining the corporeal bodies, and they form a collar of connecting channels at the base of the penis that drain by way of the superficial nodes. The superficial nodes drain to the deep inguinal nodes (those deep to the fascia lata). From there, drainage is to the pelvic nodes (external iliac, internal iliac, and obturator). Penile lymphangiographic studies demonstrate a consistent pattern of drainage that proceeds from superficial inguinal to deep inguinal to pelvic node sites without evidence of ipsilateral drainage (Cabanas, 1977, 1992). Multiple cross-connections exist at all levels of drainage, so that penile lymphatic drainage is bilateral to both inguinal areas. Metastatic enlargement of the regional nodes eventually leads to skin necrosis, chronic infection, and death from inanition, sepsis, or hemorrhage secondary to erosion into the femoral vessels. Clinically detectable distant metastatic lesions to the lung, liver, bone, or brain are uncommon and are reported to occur in 1% to 10% of cases in most large series (Staubitz et al, 1955; Riveros and Gorostiaga, 1962; Beggs and Spratt, 1964; Derrick et al, 1973; Johnson et al, 1973; Kossow et al, 1973; Puras et al, 1978). Such metastases usually occur late in the course of the disease after the local lesion has been treated. Distant metastases in the absence of regional node metastases are unusual. Carcinoma of the penis is characterized by a relentless progressive course, causing death for the majority of untreated patients within 2 years (Beggs and Spratt, 1964; Skinner et al, 1972; Derrick et al, 1973). Rarely, long-term survival occurs, even with advanced local disease and regional node metastases (Furlong and Uhle, 1953; Beggs and Spratt, 1964). No report of spontaneous remission of carcinoma of the penis is known. Five to 15 percent of patients have been reported to develop a second primary neoplasm (Buddington et al, 1963; Beggs and Spratt, 1964; Gursel et al, 1973), and one series reported secondary carcinoma in 17% of patients (Hubbell et al, 1988). Penile tumors may present anywhere on the penis but occur most commonly on the glans (48%) and prepuce (21%). Other tumors involve the glans and prepuce (9%), the coronal sulcus (6%), or the shaft (<2%) (Sufrin and Huben, 1991). This distribution of lesions may be due to constant exposure of the glans, coronal sulcus, and interior prepuce to irritants (e.g., smegma, HPV infection) within the preputial sac, whereas the shaft is relatively spared.
![]() for a discussion of benign lesions, premalignant cutaneous lesions (including Figure 34–1), and virus-related dermatologic lesions.
for a discussion of benign lesions, premalignant cutaneous lesions (including Figure 34–1), and virus-related dermatologic lesions.
Benign Lesions
Noncutaneous Lesions
Cutaneous Lesions
Premalignant Cutaneous Lesions
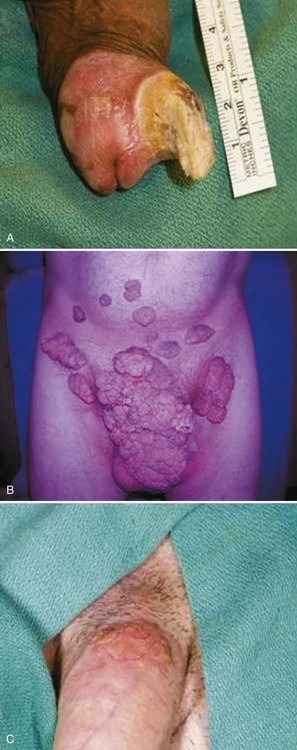
Cutaneous Horn
Pseudoepitheliomatous Micaceous and Keratotic Balanitis
Male Lichen Sclerosus (Balanitis Xerotica Obliterans)
Virus-Related Dermatologic Lesions
Human Papillomavirus in Malignant Transformation
Bowenoid Papulosis
Kaposi Sarcoma
Buschke-Löwenstein Tumor (Verrucous Carcinoma, Giant Condyloma Acuminatum)
Squamous Cell Carcinoma
Carcinoma in Situ
![]() ). Both tumors are characterized by the noninvasive changes of carcinoma in situ. Visceral malignant disease is not associated with erythroplasia of Queyrat, and subsequent case-control studies have shown no association of Bowen disease with internal malignant tumors (Anderson et al, 1973). Thus penile carcinoma in situ does not in itself warrant a specific search for internal malignant tumors. Bowen disease is characterized by sharply defined plaques of scaly erythema on the penile shaft. Crusted or ulcerated variants can occur. The appearance can be confused with bowenoid papulosis, nummular eczema, psoriasis, and superficial basal cell carcinoma. If it is not treated then invasive carcinoma may arise in about 5% of cases (Buechner, 2002). When all cases of carcinoma in situ are considered, metastasis is extremely rare but has been reported (Eng et al, 1995).
). Both tumors are characterized by the noninvasive changes of carcinoma in situ. Visceral malignant disease is not associated with erythroplasia of Queyrat, and subsequent case-control studies have shown no association of Bowen disease with internal malignant tumors (Anderson et al, 1973). Thus penile carcinoma in situ does not in itself warrant a specific search for internal malignant tumors. Bowen disease is characterized by sharply defined plaques of scaly erythema on the penile shaft. Crusted or ulcerated variants can occur. The appearance can be confused with bowenoid papulosis, nummular eczema, psoriasis, and superficial basal cell carcinoma. If it is not treated then invasive carcinoma may arise in about 5% of cases (Buechner, 2002). When all cases of carcinoma in situ are considered, metastasis is extremely rare but has been reported (Eng et al, 1995).
Invasive Carcinoma
Etiology
Prevention
Natural History
Modes of Presentation
Signs
Tumors of the Penis
• Human papillomavirus infection among men has been associated with penile carcinoma as well as with cervical dysplasia and carcinoma among female partners.
• Penile cancer is rare in developed countries and varies worldwide with age, circumcision, and hygiene practices.