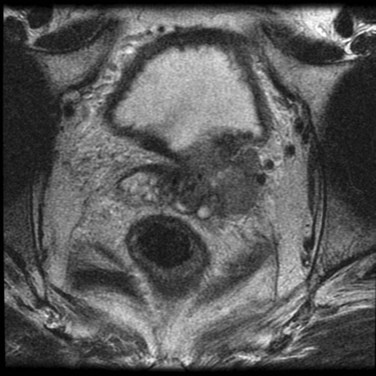
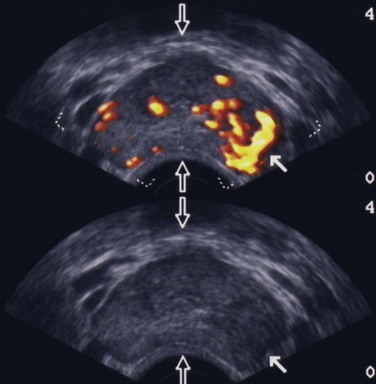
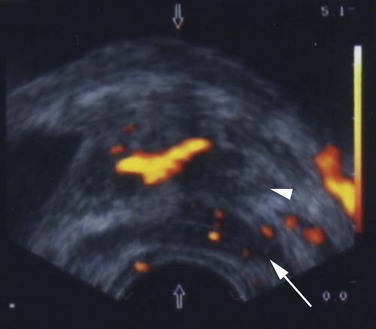
Maxwell V. Meng, MD, Peter R. Carroll, MD, MPH Traditionally, the identification of patients with locally advanced disease was based on clinical examination (e.g., digital rectal examination) and clear evidence of spread outside of the prostate capsule (clinical stage T3a), involvement of the seminal vesicles (cT3b), or involvement of adjacent organs (cT4) (Greene et al, 2002). However, the contemporary use of PSA has led to the majority of men diagnosed with prostate cancer initially having nonpalpable cancers (cT1c). Features other than clinical T stage most often contribute to the identification of men with advanced disease and a concomitant increased risk of failure after primary therapy. Thus improved methods of risk assessment have facilitated categorization of men as “high risk” and involve consideration of variables such as serum PSA level and Gleason score in addition to clinical stage. The subsequent discussion focuses on this broader definition of locally advanced disease, with inclusion of those with regional or lymph node involvement without distant metastasis (T3-4N ±M0). Multiple methods are currently available to accurately risk stratify men with prostate cancer (Partin et al, 1997; D’Amico et al, 1999; Tewari et al, 2001; Shariat et al, 2008). The Partin tables were initially constructed in the 1990s and updated in 2001, assisting in the preoperative prediction of final pathologic stage in men with clinically localized prostate cancer undergoing radical prostatectomy (Partin et al, 2001). Although clinical stage, serum PSA level, and Gleason score individually predict pathologic stage and prognosis, the combination of these three variables increases the accuracy of this assessment. These data continue to illustrate that a significant number of men thought to harbor organ-confined tumors have more advanced disease. The ability to assess pathologic stage permits better pretreatment counseling of patients and more appropriate selection of therapy and consideration of those with more advanced disease for novel clinical trials. The most important pathologic criteria predicting prognosis after radical prostatectomy are Gleason score, surgical margin status, and presence of non-organ-confined disease (e.g., extracapsular extension, seminal vesicle invasion, lymph node involvement). In addition, to help predict pathologic stage, biopsy information has been incorporated into models estimating cancer outcomes, primarily PSA- or biochemical-free survival, after treatment. Whereas biochemical-free survival is a frequent end point of current prostate cancer studies, it merely represents an intermediate surrogate outcome variable; further trials and studies are necessary to confirm the utility of biochemical recurrence as a marker for reduced prostate cancer-specific survival after treatment. The most widely used tools to predict disease recurrence after local therapy are the nomograms developed by Kattan and colleagues (1998, 2000). In all pretreatment nomograms, clinical stage, biopsy Gleason grade, and pretreatment serum PSA level are incorporated to predict the continuous risk of disease progression after definitive local therapy; models for radiation therapy (RT) also include data on use of androgen deprivation (AD), total radiation dose, and combination treatment (i.e., external beam and permanent brachytherapy). The radical prostatectomy nomogram was based on nearly 1000 patients with clinically localized disease (T1c-T3aNXM0); thus this model is not applicable for those men with evidence of seminal vesicle involvement or regional spread. Key Points: Risk Assessment To simplify risk stratification, men with prostate cancer can be grouped into fewer categories but with maintenance of ability to predict disease behavior and response to intervention. D’Amico and colleagues (1998) defined patients at low, intermediate, and high risk for biochemical failure, still based on pretreatment disease characteristics (clinical stage, PSA value, Gleason score). Recurrence after treatment may be due to unrecognized micrometastatic disease or persistence of locoregional disease. Other variations of simplified risk stratification have been developed and validated, with inclusion of features such as ethnicity and pathologic findings (Moul et al, 2001; Cooperberg et al, 2005). The Cancer of the Prostate Risk Assessment score, ranging from 0 to 10, provides another method to assess risk with each 2-point increase in score doubling the risk of recurrence after prostatectomy. Although men with “low-risk” disease generally do well and are unlikely to have biochemical recurrence, men in the intermediate- and high-risk groups may have widely discrepant outcomes. Therefore men in these groups benefit from more modern and accurate risk prediction models and nomograms (Mitchell et al, 2005). Although observations on traditional gray-scale ultrasonography can identify extraprostatic disease, the general ability to improve cancer staging is limited (Figs. 107-1 and 107-2). Directed biopsy of the seminal vesicles or prostate capsule can be obtained to confirm cT3 disease. Smith and colleagues (1997) found that transrectal ultrasonography did not improve the ability to stage prostate cancer; the area under the curve was .69 and .74 for transrectal ultrasonography in predicting extracapsular extension and seminal vesicle invasion, respectively, compared with .72 and .69 for digital rectal examination, respectively. Salo and colleagues (1987) reported high sensitivity (86%), specificity (94%), positive predictive value (92%), and negative predictive value (89%) of ultrasonography in predicting extracapsular extension in patients undergoing radical prostatectomy. However, more contemporary studies suggest otherwise, with lower sensitivities (23% to 66%), specificities (46% to 86%), positive predictive values (50% to 62%), and negative predictive values (49% to 69%). The inaccuracy of ultrasound staging for prostate cancer is likely to be the result of significant interobserver variability, often subtle signs of extraprostatic spread, and lower-volume tumors currently diagnosed. In general, transrectal ultrasonography understages rather than overstages prostate cancer. Newer ultrasound techniques such as color and power Doppler studies are under investigation to determine if observations of abnormal blood flow can increase the ability to detect and stage prostate tumors. (Courtesy of Dr. Katsuto Shinohara.) (Courtesy of Dr. Katsuto Shinohara.) Endorectal magnetic resonance imaging (MRI) uses a magnetic coil placed in the rectum to achieve better visualization of the zonal anatomy of the prostate and may delineate subtle distinctions between T2a/b disease and T3 disease (Fig. 107–3). However, use of MRI, alone or in combination with magnetic resonance spectroscopy (MRS), for tumor staging remains controversial. Variable sensitivities (13% to 91%) and specificities (49% to 97%) have been reported for predicting extracapsular extension, in part attributed to variability in interpreting MRI and lack of uniform diagnostic criteria. In a cohort of 336 men with more than three cores involved with cancer, abnormal findings on digital rectal examination, and PSA level higher than 10 ng/mL undergoing radical prostatectomy, the specificity of MRI in predicting pT3 disease was 95% (Cornud et al, 2002). Thus the use of MRI may be best limited to those patients with higher-risk features. It is clear that traditional clinical and pathologic parameters are limited in their ability to accurately predict local extent of tumor before treatment. Given the importance of assessing true pathologic stage, novel markers of advanced disease are necessary. Chu and colleagues (2003) used comparative genomic hybridization in primary prostate specimens and identified chromosomal regions potentially useful in discriminating between organ-confined and locally advanced tumors (Table 107–1). Prediction of stage by a model incorporating six aberrations in a stepwise fashion was accurate in 91.1% of cases. Further metabolic, genetic, and proteomic signatures will likely better define and discriminate between organ-confined and non–organ-confined prostate cancer (Ashida et al, 2004; Paris et al, 2004; Mehra et al, 2007; Sreekumar et al, 2009). Chromosomal rearrangements involving the androgen-regulated gene TMPRSS2 and the ETS family genes are associated with higher pathologic stage, whereas the glycine-metabolite sarcosine was more often elevated in metabolomic profiles of men with metastatic prostate cancer. Table 107–1 Chromosome Abnormalities Associated with Pathologic Stage Within the Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE), Cooperberg and colleagues (2003) observed a significant decrease in the fraction of men presenting with high-risk disease characteristics, as defined by D’Amico criteria, from 40.9% in 1989-1990 to 14.8% in 2001-2002. However, alterations in cancer screening and use of PSA are likely to account for this reduction, with significantly fewer men presenting with elevations in PSA level higher than 20 ng/mL (32.8% to 7.2%); indeed, currently, higher Gleason grade defines a larger number of high-risk patients (61.5%). Overall, the presence of clinically advanced disease (i.e., T3-4) decreased from 11.8% to 3.5%, with a decline from 32.3% to 21.9% in the high-risk group during the observation period. In men undergoing radical prostatectomy, the number of men with clinical evidence of locally advanced disease, as defined by clinical stage (T3), has declined from 25.3% in 1987 to 2.8% in 2001 (Ward and Zincke, 2003). Although the use of PSA screening can account for part of this change, the more selective application of surgery and improved techniques of RT may also explain the decline in patients with cT3 disease undergoing prostatectomy. Within reports of men undergoing radical prostatectomy, the fraction of men with pT3 disease has decreased; these are highly selected patients with largely clinically localized disease at the time of treatment (Table 107–2). Roehl and colleagues (2004) reported that pathologically advanced disease decreased from 39% (1983-1991) to 31% (1992-2003). In men undergoing radical prostatectomy alone at the Cleveland Clinic, the overall rate of extracapsular extension declined from 65.8% (1987-1989) to 25.2% (2000-2001), and this trend was true for all clinical stages of tumor (T1c-T2b/c), as well as for pretreatment variables (PSA value, Gleason score). Key Points: Trends in Incidence and Treatment The National Comprehensive Cancer Network (2004, 2009) provided updated decision trees for men with prostate cancer to aid in treatment selection. High risk of recurrence includes men with clinically advanced disease, both cT3a and cT3b-T4. Bone scans are indicated in these men, as well as in those with PSA greater than 20 ng/mL or Gleason score greater than or equal to 8; pelvic computed tomography (CT) or MRI is also indicated for cT3-T4 or if the calculated probability of lymph node involvement is greater than 20%. In general, radical prostatectomy with pelvic lymphadenectomy is reserved for those high-risk men with low-volume tumors that can be completely excised. The alternative treatment strategy for these men with locally advanced disease and higher risk of biochemical failure is AD therapy combined with RT. The shift in treatment is also reflected within the CaPSURE database (Meng et al, 2005). A total of 6074 men with prostate cancer (clinical stage lower than T3a,N0,M0) was stratified according to risk group, of whom 26% were high risk. Fewer men in this group underwent radical prostatectomy compared with the low-risk cohort, with older age, advanced disease, and increased number of comorbidities significant predictors of treatment (external beam radiation or AD therapy) in multivariate modeling. In addition, more than half of high-risk men receiving radiation therapy also received AD, significantly more than in the low- and intermediate-risk groups (P < .0001). Active surveillance, with deferred treatment when necessary, is becoming a viable treatment alternative in men with prostate cancer. Multiple studies have suggested that cancer-specific mortality is low and primarily associated with higher-grade and higher-stage disease. Even in those low-risk cancers, however, an increased and unexpected progression of disease and associated prostate cancer mortality may be seen during an extended period (Johansson et al, 2004). In men with higher-risk disease characteristics, it has been recognized that disease progression occurs more rapidly and that some form of intervention is typically warranted in healthy patients. Few reports address the specific question of outcome in men with locally advanced cancers merely observed for a prolonged period. Older studies such as that from Nesbit and Plumb (1946) have little relevance to current disease management. Other studies have included only a small number of patients with higher clinical stage. A range of clinical progression (22% to 75%), local progression (22% to 84%), and development of distant metastases (27% to 56%) has been reported during 5 and 10 years of follow-up. Overall survival is reported from 10% to 92% at 5 years and 14% to 78% at 10 years for patients who harbor cancers of high grade or stage. The Veterans Administration Cooperative Urological Research Group (VACURG) study reported a 58% overall 5-year survival in 248 men with clinical stage III disease treated with placebo. Comparable survival outcomes were reported within the Medical Research Council study (Adib et al, 1997) randomizing previously untreated patients with locally advanced, nonmetastatic disease (n = 501) to early or delayed AD therapy (castration or luteinizing hormone-releasing hormone [LHRH] agonist). The median time to clinical progression and death from prostate cancer in the 244 patients receiving delayed treatment was 10 months and 48 months, respectively. In the analysis from Chodak and colleagues (1994), grade 3 tumors were significantly associated with disease-specific mortality (risk ratio, 10.04) in men treated conservatively, compared with low-grade (grade 1) cancers. The 10-year disease-specific survival was 87% in men with grade 1 or grade 2 tumor and 34% with grade 3 tumor, with metastasis-free survival of 81% for grade 1, 58% for grade 2, and 25% for grade 3 diseases. Johansson and colleagues (1997) prospectively observed 642 men with prostate cancer diagnosed between 1977 and 1984, with any stage of disease. Of those with clinically localized disease, 11% of men died of prostate cancer with corrected 15-year survival comparable for those who received initial and deferred treatment. Conversely, the corrected 15-year survival was 57% in patients with locally advanced cancer. Approximately half of men had well-differentiated tumors, and only 6% of these men died of prostate cancer. Death from prostate cancer increased with moderately differentiated (17%) and poorly differentiated (56%) disease. These data are summarized in Table 107–3. Albertsen and colleagues (1998) reported the long-term outcomes of watchful waiting in 767 men identified from the Connecticut Tumor Registry with clinically localized prostate cancer (1971-1984). The 15-year cancer-specific mortality in men with Gleason sum 6 was 18% to 30%, compared with the 25% to 59% risk of death from other causes. The chances of death from prostate cancer increased with Gleason score 7 (42% to 70%) and 8 to 10 (60% to 87%). It is likely that a significant proportion of this cohort had non–organ-confined disease because none of the men underwent PSA testing and therefore probably had more advanced stages of disease compared with contemporary cohorts. In contrast to the report from Johansson and colleagues (2004), the annual mortality rate from low-grade prostate cancer appears to remain stable beyond 15 years after diagnosis (Albertsen et al, 2005) (Fig. 107–4). Men with high-risk prostate cancer including those with locally advanced disease are at significant risk of disease progression and cancer-specific death if left untreated. (Modified from Albertsen PC, Hanley JA, Fine J: 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095–101.) Several series report outcomes of radical prostatectomy for clinical stage T3 tumors (Table 107–4). In examining all reports, overall survival ranges from 64% to 96% at 5 years, 12.5% to 72% at 10 years, and 20% to 51% at 15 years after treatment. Earlier data reflected less accurate risk assessment and a potentially greater number of patients with unsuspected lymph node metastases and associated earlier progression and death. Less variability exists in more contemporary cohorts, in which the cancer-specific survival rates are 85% to 92% and 79% to 82% at 5 and 10 years, respectively, regardless of adjuvant therapy. Pound and colleagues (1999) found that a reasonable outcome was possible after prostatectomy for clinical stage T3a disease, with a 52% 8-year, recurrence-free survival. Similarly, Ward and Zincke (2003) reported 5- and 10-year cancer-free survival of 60% and 44%, respectively, without application of adjuvant AD. Freedom from local or systemic disease at 5 and 15 years after surgery was 73% and 67%, respectively (Ward et al, 2005). In the 812 patients from the series of Lerner and colleagues (1995), 10-year cancer-specific survival was 80%, with only 31% of men with clinical stage T3 disease dying of prostate cancer 15 years after radical prostatectomy. It is unclear, however, whether surgical intervention improves survival compared with alternative treatment strategies. Another interesting observation from the Mayo Clinic was that clinical overstaging was identified in 27% of patients, consistent with other reported rates of 7% to 26%, suggesting that uniformly excluding patients from prostatectomy on the basis of clinical staging may not be appropriate (Ward et al, 2005). Gerber and colleagues (1997) reviewed results in 298 men with clinical stage T3 disease undergoing radical prostatectomy and pelvic lymphadenectomy. Although the overall 10-year cancer-specific survival was 57%, an apparent benefit of radical prostatectomy in some men with locally advanced disease was suggested by the increased survival (70%) in those who actually underwent prostatectomy and lymph node dissection. Many men with clinical stage T3 disease have regional spread and may not benefit from prostatectomy; however, select patients (e.g., lower-volume disease) may benefit because local control may be achieved in most, and complete cancer excision is possible in some men. Biochemical progression after radical prostatectomy is difficult to assess, given the frequent use of adjuvant therapy (e.g., RT or AD). Without the use of secondary treatment, 5-year biochemical relapse is higher than 60% (van den Ouden et al, 1998). In other series with variable use of adjuvant therapy, 5- and 10-year biochemical progression was observed in 42% to 49% and 59% to 62%, respectively. The impact of adjuvant therapy may be minimal with respect to clinical progression (i.e., biopsy-proven local recurrence or objective distant metastasis) after radical prostatectomy. Rates of clinical progression at 5, 10, and 15 years are 12% to 45%, 39% to 49%, and 50% to 71%, respectively. As discussed, pathologic stage after radical prostatectomy provides important prognostic information and is a powerful predictor of outcome, considering all clinical and pathologic factors (Fig. 107–5). The presence of focal and established extracapsular extension increases the rate of clinical progression from 7% for organ-confined disease to 18% and 35%, respectively, at 5 years. Patients with evidence of seminal vesicle invasion or lymph node metastasis are highly likely to develop clinical progression (86% and 95%, respectively) after radical prostatectomy. In men undergoing radical prostatectomy for clinically organ-confined disease, Hull and colleagues (2002) reported 5-year PSA-free survival of 95%, 76%, 37%, and 18% for men with pathologic organ-confined disease, extracapsular extension, seminal vesicle invasion, and positive lymph nodes, respectively. In multivariate analysis, pathologic parameters increasing the relative risk (RR) for progression included extracapsular extension (RR, 2.17 to 2.72), seminal vesicle involvement (RR, 2.61), and lymph node involvement (RR, 3.31). The presence of a positive surgical margin was associated with the greatest relative risk (4.37; range, 2.90 to 6.58). This reinforces the concept that complete surgical removal of all prostatic tissue, regardless of clinical or pathologic stage, should be accomplished when prostatectomy is undertaken. Even with seminal vesicle invasion, however, men without concomitant lymph node involvement can achieve 15-year cancer-specific survival and biochemical relapse-free rates of 81% and 32%, respectively (Secin et al, 2006). (Modified from Bianco FJ Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”). Urology 2005;66:83–94.) Biochemical progression after prostatectomy for pathologically advanced tumors depends on the definition applied. Five-year rates of biochemical recurrence have been as high as 65% to 72% when a PSA threshold of 0.2 ng/mL is used; less stringent thresholds such as 0.4 ng/mL may yield recurrence rates of 26% at 5 years and at least 50% at 10 years. Seminal vesicle involvement not only increases the risk of biochemical recurrence but also significantly increases the risk of local recurrence after radical prostatectomy. With prolonged follow-up, Hawkins and colleagues (1995) reported local recurrence in nearly half of patients. This risk appears to be lower in contemporary series of patients because of improved selection of patients for surgery, improved surgical technique, and earlier use of secondary treatment either as adjuvant therapy or at the time of biochemical relapse. Predictive models have permitted preoperative estimation of PSA-free survival after prostatectomy. Patients with higher biopsy Gleason score and more elevated serum PSA level are at increased risk of failure after surgery. Overall, the actuarial PSA-free survival after surgery in high-risk men is approximately 50% at 5 to 7 years. The validity of the Kattan preoperative nomogram was examined in a community-based cohort of patients undergoing radical prostatectomy (Greene et al, 2004). Interestingly, although the overall concordance index (.68) supported the applicability of the tool to a broad spectrum of patients, the nomogram underestimated recurrence in lower-risk men and overestimated recurrence in men at higher calculated risk. Indeed, up to half of men with Gleason sum 8 to 10 or higher PSA level (>20 ng/mL) may achieve prolonged disease-free survival with prostatectomy alone. Further refinements of risk assessment and novel markers will help determine which high-risk men and locally advanced tumors truly benefit from aggressive surgical intervention, either alone or in combination with other therapy. To improve outcomes of radical prostatectomy in men with locally advanced or high-risk tumors, several investigators have assessed the use of neoadjuvant AD (NAD) before radical prostatectomy. In 1944 Vallet reported performing radical perineal prostatectomy after orchiectomy in a 59-year-old man with prostate cancer. Others reported the use and efficacy of diethylstilbestrol (DES) before surgery. In 1964 Scott evaluated the results of NAD in 31 men who were observed for 10 years. The majority of patients (52%) were alive and free of clinical recurrence. The prospective randomized trials of NAD in patients with stage cT1-T3 prostate cancer before radical prostatectomy are summarized in Table 107–5. Table 107–5 Prospective Randomized Trials of Neoadjuvant Androgen Deprivation in Patients with Prostate Cancer (Stage cT1-T3) before Radical Prostatectomy Key Points: Radical Prostatectomy Various measures of outcome have been evaluated in these studies including changes in digital rectal examination (clinical stage), appearance of tumor on imaging, detection of micrometastatic or circulating cancer cells, and pathologic features such as T stage, surgical margin and lymph node status, and histopathologic changes. Ultimately, the utility of AD must be assessed by its impact on disease-specific survival or its current surrogate, biochemical-free survival. It is clear that NAD affects tumor behavior and biology, evaluable by changes in metabolic patterns of atrophy on MRS, lowering of serum PSA level, and histologic atrophy (fibrosis, vacuolization, glandular collapse). Studies have demonstrated that significant reductions in prostate volume (30% to 50%), tumor volume, and PSA level (90%) are consistently noted, with maximal effects occurring during the first 2 months of treatment. However, conflicting results have been reported with respect to pathologic downstaging after NAD in patients with clinical stage T3 cancer. Overall, only 20% of such patients have organ-confined disease at the time of radical prostatectomy despite clinical downstaging in 32% to 90%. Schulman and colleagues (2000) described 402 patients with cT2-T3N0M0 tumors randomized to either radical prostatectomy alone or 3 months of total androgen blockade followed by surgery. Pathologic downstaging was seen more frequently in the neoadjuvant group (15%) than in the prostatectomy-alone group (7%; P < .01); however, in men with cT3 disease, there was no difference in pathologic downstaging (P = .18) and the incidence of lymph node metastases (P = .36) in the neoadjuvant and surgery-alone cohorts. Thus whereas clinical downstaging may occur frequently, pathologic downstaging is significantly less common after NAD, ranging between 8% and 31%. Most studies do not show decreased seminal vesicle invasion (Table 107–6). Similarly, rates of lymph node metastases are not altered, with the incidence varying between 3.3% and 16% in both the prostatectomy-alone and NAD groups. Table 107–6 Pathologic Findings from Studies of Neoadjuvant Androgen Deprivation (NAD) in Patients with cT1-T3 Prostate Cancer before Radical Prostatectomy Klotz and colleagues (2003) provided long-term follow-up (median, 6 years) of a prospective randomized trial comparing 3 months of NAD before prostatectomy and surgery alone. There was no overall benefit of NAD as measured by biochemical recurrence (34% to 38%), although in the subgroup of men with PSA level above 20 ng/mL, those receiving NAD had greater PSA-free survival (53%) compared with those undergoing prostatectomy alone (35%; P = .015). The Southwest Oncology Group (SWOG) Study 9109 was a phase II feasibility study of 16 weeks of goserelin and flutamide before radical prostatectomy in men with T3-T4N0M0 prostate cancer (Powell et al, 2002). Of the 55 patients who underwent prostatectomy, 31% had seminal vesicle invasion and 19% had lymph node metastasis. Organ-confined disease was identified in 67% of cases. At median follow-up of 6.1 years, 5-year progression-free and overall survival estimates were 70% and 90%, respectively. The study demonstrated the feasibility of 4 months of AD before surgery, with acceptable morbidity, and outcomes comparable with RT for this population of patients. Studies have also examined the incidence of positive surgical margins in those with cT3 disease. Witjes and colleagues (1997) reported similar rates of positive margins in men receiving NAD and those undergoing radical prostatectomy alone (43% and 59%, respectively; P = .14). Van Poppel and colleagues (1995) did not find a statistically significant difference between these groups with respect to positive margins—41.3% in the neoadjuvant cohort and 44% in the surgery-alone group. SWOG 9109 reported positive surgical margins in 30% of patients. In contrast, the randomized and nonrandomized studies of NAD in men with lower clinical stage (cT1-T2) clearly demonstrate a reduction in the rate of positive surgical margins. This advantage has not translated into improved long-term PSA-free survival. For locally advanced tumors (specifically cT3), current data, both retrospective and prospective, do not support a significant benefit of neoadjuvant androgen deprivation before surgery (Table 107–7). Table 107–7 Neoadjuvant Androgen Deprivation (NAD) Therapy for 3 Months before Radical Prostatectomy in Patients with Localized Prostate Cancer: Impact on PSA Recurrence after Surgery The apparent lack of benefit of NAD before radical prostatectomy, in men with both lower-stage and locally advanced prostate cancer, may be due to a number of factors. It has been suggested that 3 months of AD may be insufficient to cause an improvement in disease-free survival compared with prostatectomy alone. Meyer and colleagues (1999) followed 680 men treated with radical prostatectomy, of whom 292 received NAD. Although there was no difference in risk of biochemical recurrence between the two groups, patients receiving both LHRH agonist and antiandrogen for more than 3 months had a significantly lower risk of PSA failure than did those treated with surgery alone (hazard rate [HR], 0.52; 95% confidence interval [CI], 0.29 to 0.93). Subsequently, the Canadian Urologic Oncology Group (Gleave et al, 2001) randomized patients with clinically localized disease to either 3 or 8 months of NAD before radical prostatectomy. In the 500 patients with pathologic staging, positive surgical margins were identified in 23% of the 3-month group and 12% of the 8-month group (P = .011); organ-confined disease was found in 68% and 80% of the 3- and 8-month groups, respectively (P = .0019). In addition, the rate of non–specimen-confined or lymph node extension was greater in the 3-month group (25.6%) compared with the 8-month group (12.6%). However, despite evidence of continued biochemical and pathologic regression of the tumor between 3 and 8 months, no significant difference in PSA recurrence rates was observed at 4 years. The role of chemotherapy in the treatment of prostate cancer has primarily been limited to men with the most advanced disease. Taxanes, alone or in combination with other agents, have proved effective in those with hormone-refractory prostate cancer, with significant PSA declines (>50%) in more than half of patients and measurable disease response in 28% to 75% (Oh, 2004). Mitoxantrone plus low-dose steroids has shown benefit in pain relief compared with steroids alone and is approved for use in hormone-refractory disease (Tannock et al, 1996; Kantoff et al, 1999). On the basis of these observations, interest has increased in earlier use of chemotherapy in high-risk patients or those with locally advanced disease. These studies are summarized in Table 107–8. Table 107–8 Trials of Chemotherapy, Hormonal Therapy, and Combined Chemo-Hormonal Therapy before Radical Prostatectomy Pettaway and colleagues (2000) treated 33 higher-risk patients with a 3-month combination regimen consisting of two 6-week cycles of ketoconazole and doxorubicin alternating with vinblastine and estramustine. In addition, concurrent AD was accomplished with an LHRH agonist and antiandrogen. Lower pathologic stage was found in 18% of patients with cT3 disease, and the overall positive surgical margin rate of only 17% suggests that the regimen may have improved the ability to resect the cancer above that achieved with NAD alone. A second report of chemotherapy and hormone therapy before surgery combined 4 to 6 months of NAD with paclitaxel, estramustine, and carboplatin in patients with androgen-dependent, high-risk prostate cancer (Konety et al, 2004). No patients were downstaged to pT0 after neoadjuvant therapy, and organ-confined disease was reported in 36%, with a 22% positive surgical margin rate. Overall, 45% remain without biochemical relapse, defined as PSA level above 0.05 ng/mL on three occasions, at median follow-up of 29 months, and no patient has had documented local recurrence. At 43 months follow-up, Chi and colleagues (2008) reported a 3% complete pathologic response rate and distant relapse rate of 30% in patients treated with NAD and docetaxel. Other centers have used chemotherapy without concurrent NAD. At the Cleveland Clinic, 16 patients with locally advanced disease (clinical M0) underwent three cycles of oral estramustine and etoposide and subsequent surgery (Clark et al, 2001). Although grade 3 or grade 4 toxicity was seen in 34% of patients, surgery was not delayed in any individual and surgical outcomes were comparable with those in series of prostatectomy without neoadjuvant therapy. With a median follow-up of 14 months, 88% were free of disease and the early PSA failures occurred in the two patients with lymph node metastases. Single-agent docetaxel is well tolerated before radical prostatectomy, with minimal toxicity (Oh et al, 2001; Dreicer et al, 2004). No cases of complete pathologic response were observed, but no lymph node metastases were identified in one series, whereas the other reported 11% with organ-confined disease. Two thirds and 24% of patients, respectively, experienced a more than 50% reduction in PSA level in response to docetaxel alone. Similar observations have been made using a novel nanoparticle-based formulation of paclitaxel, with no complete pathologic responses (Shepard et al, 2009
Definition
Contemporary Risk Assessment
Imaging Modalities
Novel Markers
CHROMOSOME ABERRATION (INDEPENDENT OR IN COMBINATION)
P VALUE
−8p
.001
−8p/−10q23 → qter
.002
−10q25 → qter
.029
−6p21
.031
−6q24 → qter
.031
−18cen-q12
.035
−5q31/−10q24 → qter
.04
−5q31 → qter/−8p
.04
−6p21/−10q24 → qter
.04
−6p12 → pter/−6q24 → qter
.04
−6q25 → qter/−11q23 → qter
.04
+7p11/−10q24 → qter
.04
−8p/−15q22
.04
−8p/−11q24 → qter
.42
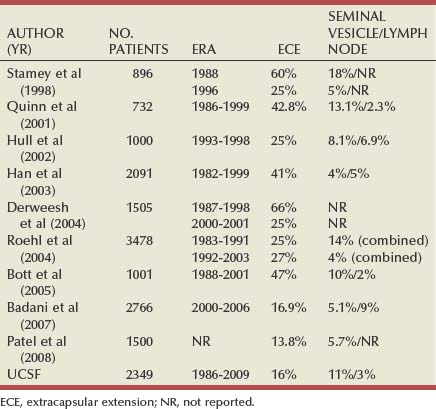
Trends in Incidence and Treatment
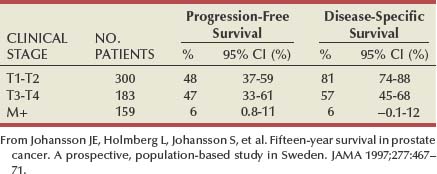
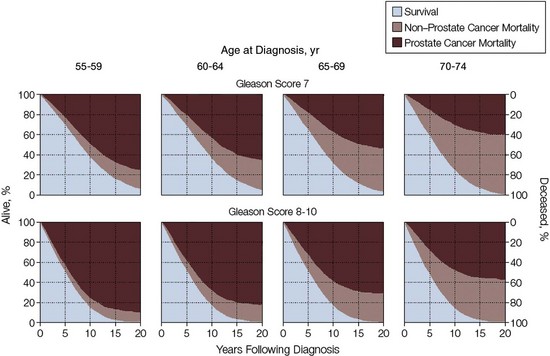
Natural History
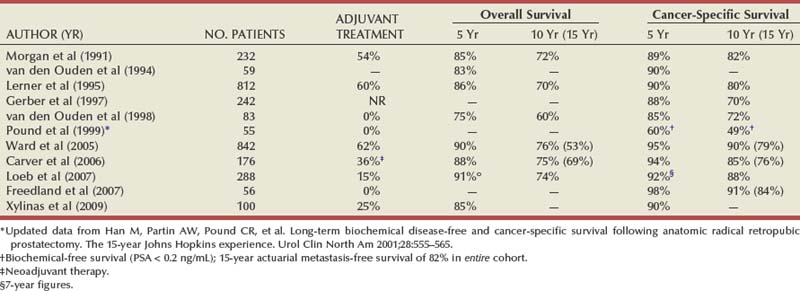
Radical Prostatectomy
Surgery for Clinical Stage T3 Prostate Cancer
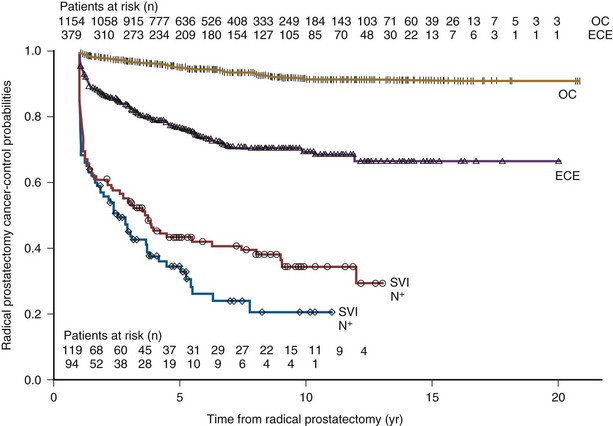
Outcomes of Prostatectomy for Pathologically Advanced Disease
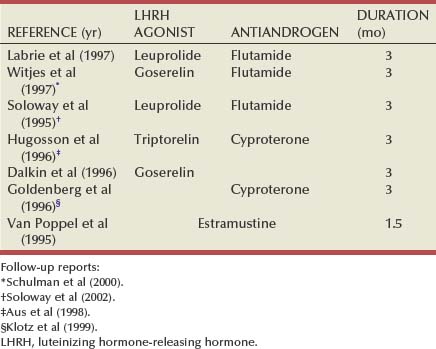
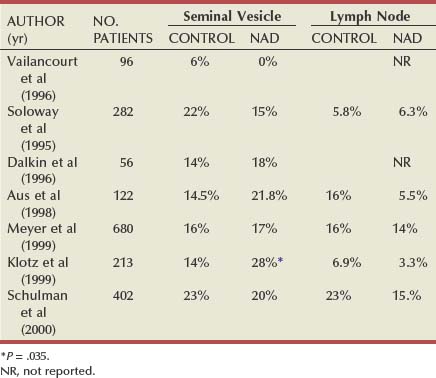
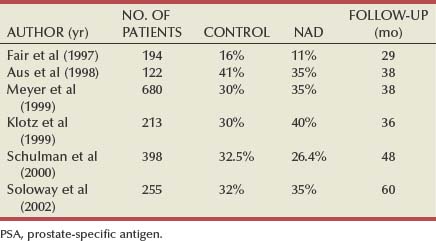
Neoadjuvant Androgen Deprivation
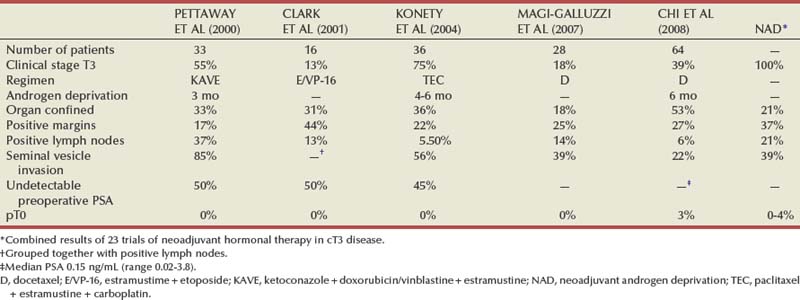
Neoadjuvant Chemotherapy and Chemotherapy-Hormone Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Treatment of Locally Advanced Prostate Cancer
• Risk assessment is best performed by a combination of serum PSA level, T stage, cancer grade, and extent of cancer on biopsy.
• Radical prostatectomy alone can result in cancer-free survival in at least half of men at 8 to 10 years despite clinically advanced disease.