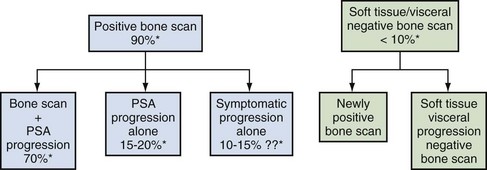
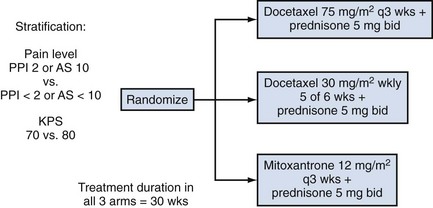
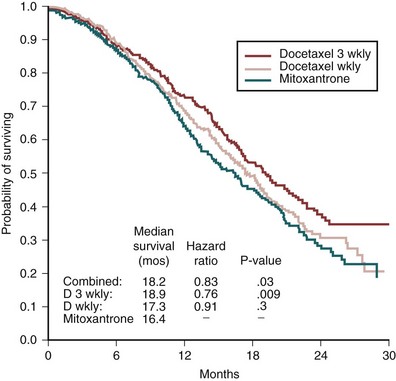
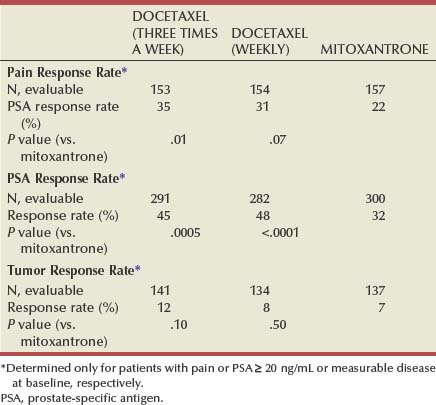
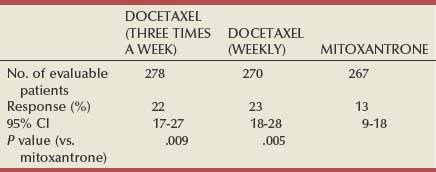
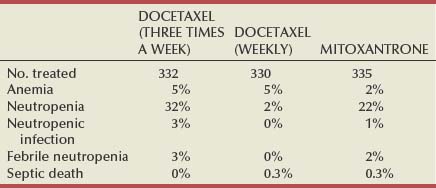
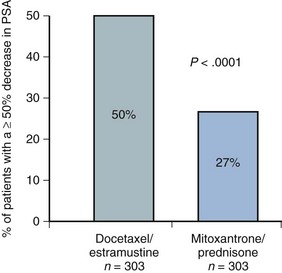
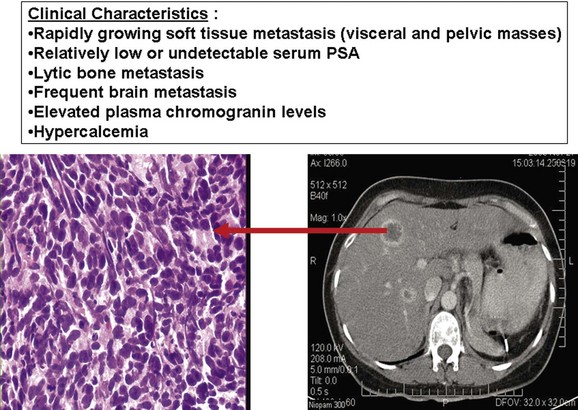
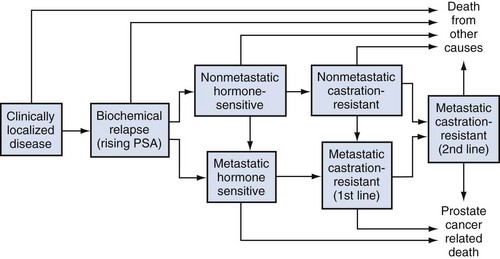
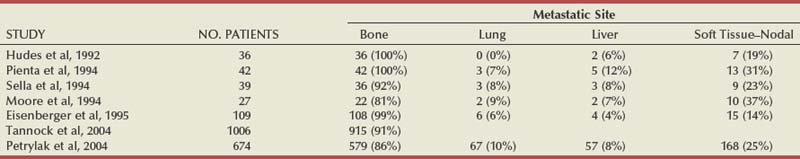
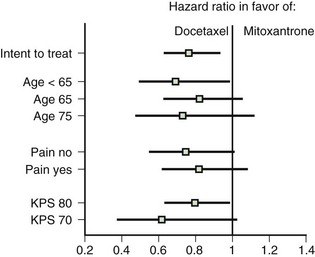
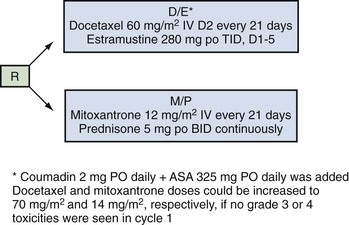
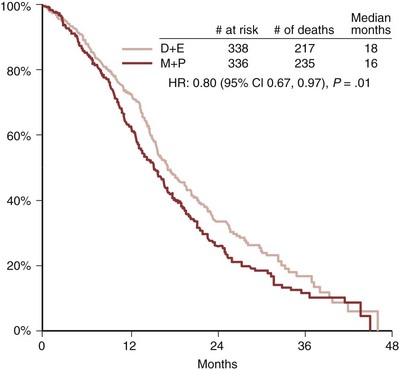
Emmanuel S. Antonarakis, MD, Michael A. Carducci, MD, Mario A. Eisenberger, MD During the past several decades, endocrine manipulations developed to inhibit hormone-dependent prostate cancer growth and differentiation have constituted the basic strategy for the systemic control of prostate cancer. Suppression of gonadal testosterone is the central principle of androgen-deprivation therapy (ADT), and this represents one of the most effective systemic palliative treatments known for solid tumors. Although it is extremely effective initially, virtually all patients eventually develop clinical evidence of treatment resistance. The outcomes with endocrine therapy have not changed significantly during the past few decades. Progression-free and overall survival figures of patients with metastatic disease with various methods of ADT have ranged from 12 to 20 months and 24 to 36 months, respectively (Leuprolide Study Group, 1984; Crawford et al, 1989; Denis et al, 1993; Eisenberger et al, 1998). Whereas somewhat longer survival times are reported in the more recent studies, this is most likely due to a “lead time” effect observed in contemporary populations of patients. The development of hormone resistance (i.e., cancer progression despite castrate levels of serum testosterone) is virtually a universal issue that affects all patients treated with ADT. Undoubtedly, further improvement in the outcome of patients with metastatic castration-resistant prostate cancer (CRPC) rests on the use of nonhormonal approaches that can effectively control the growth of the disease. Progress in cell and molecular biology during the past decade has also enhanced our understanding of the mechanisms involved in the progression of prostate cancer, and this may provide the opportunity for rational planning of the appropriate timing of systemic therapeutic intervention with the objective of preventing or delaying progression of disease to lethal proportions. Cancer cells demonstrating the castration-refractory phenotype can be identified during early stages of development of prostate cancer. Somatic alterations of the androgen receptor are frequently observed in patients with evidence of disease progression after androgen deprivation. It has also been demonstrated that during cancer progression, in the absence of androgens, a molecularly altered androgen receptor can still undergo ligand-dependent activation by other hormones such as estrogens and progestational agents and non–ligand-dependent activation by growth factors and cytokines (Feldman and Feldman, 2001; Gelmann, 2002; Nelson WG et al, 2003). The observation that the androgen receptor can still be activated even after long-term gonadal ablation suggests that it continues to play an important role in prostate cancer growth and may indeed be a reasonable target for treatment in patients with androgen-independent disease. In the presence of androgens, prostatic cancer growth is based on a cell proliferation rate that exceeds that of cell death (Isaacs et al, 1992). Androgen ablation primarily affects the cell death rate by inducing a swift apoptotic cascade. As the tumor progresses the threshold of apoptosis progressively rises to a point at which cell proliferation exceeds cell death (Berges et al, 1995). This results in the accumulation of endocrine-independent cells that eventually dominate the biologic behavior of prostate cancer in late stages. Preclinical data suggest that the relatively low growth fraction expressed by adenocarcinoma of the prostate (compared with other common tumor types) may be a determining factor to explain the relative insensitivity to conventional cytotoxic chemotherapy. The proliferation rate of prostate cancer cells, which is directly proportional to the growth fraction, appears to increase with tumor progression especially after androgen ablation. Cell proliferation antigens, such as Ki-67 expressed by cycling cells (Cattoretti et al, 1992), may have important prognostic and therapeutic implications because most conventional cytotoxic chemotherapeutic agents available are usually more effective in tumors with high proliferative rates such as lymphomas, small cell lung carcinomas, and germ cell tumors of the testis. Changes in differentiation pathways in prostate cancer have been increasingly emphasized, particularly in the form of neuroendocrine/anaplastic cells (diSant’Agnese, 1995). Evolving experience with cytotoxic chemotherapy suggests that this aggressive clinical entity may be responsive to treatment regimens frequently employed for comparable tumors at other sites with similar phenotypic characteristics, such as small cell carcinoma of the lung. There is strong evidence to support the relationship between prostate cancer growth and various peptide growth factors (Djakiew et al, 1991; Steiner, 1993; Hofer et al, 1995; Sherwood et al, 1998; Kaplan et al, 1999; Nelson WG et al, 2003). Peptide growth factors may also exert their effects through the activation of the androgen receptor. Androgens are capable of inducing stromal production of various growth factors that could replace the androgen requirements for cell growth and differentiation (Lee, 1996). In addition, cytokines released primarily by stromal cells, such as interleukin-6, may also be important in the pathogenesis of prostate cancer. Indeed, small molecule inhibitors and other modalities of treatment (e.g., monoclonal antibodies) are being actively designed to target intracellular pathways associated with the expression of various growth factors and their receptors. Such strategies have involved inhibition of receptor tyrosine kinase activity and other intracellular molecular pathways of signal transduction as well as other critical pathways of cell growth and survival. Conventional staging criteria, such as the TNM staging system, do not describe the extent of disease beyond a simple anatomic classification. Treatment practices result in the creation of different disease states as described by Scher and colleagues (2008). This system allows classification of patients in a more clinically applicable fashion and is increasingly being used throughout the literature. Figure 110–1 illustrates the natural history of prostate cancer relative to treatment practices and identifies the various paradigms according to the response status to different therapies. Throughout this chapter prognostic and therapeutic considerations are largely based on the concepts proposed by this classification. A complete disease evaluation is required to estimate the outcome and to make therapeutic decisions. Critical baseline components include extent of disease, mode and site of progression (rising prostate-specific antigen [PSA] level alone, new bone metastasis, visceral and nodal metastasis), presence or absence of symptoms, and response to prior endocrine treatment. Regular monitoring with serial bone scintiscans and serum PSA levels during hormone treatment provides important information in patients demonstrating evidence of disease progression while they are receiving hormone therapy. Table 110–1 describes the postprogression survival of patients with distant metastatic disease who demonstrated progression of disease while on hormone therapy in a large prospective trial (Eisenberger et al, 1995). The data shown in this table illustrate the broad range in postprogression survival according to the mode of progression (PSA only vs. bone scintiscan progression) and according to the extent of bone metastases. This observation highlights the importance of establishing precisely the predominant mode of progression in these patients and underlines the need for serial determinations of all laboratory, clinical, and radiologic data. It also provides critical information for the interpretation of subsequent clinical trials with regard to time-dependent outcomes, such as time to disease progression and survival. Usually the first manifestation of disease progression after hormone therapy is a rising serum PSA level. In patients with metastatic disease, a rise in serum PSA level precedes evidence of advancing disease in the bone scintiscan, and during this time patients may remain relatively asymptomatic (Eisenberger et al, 1995). Routine evaluation of serum testosterone levels may provide important information for the choice of treatment. This is especially important when there are reasons to suspect treatment noncompliance or if the choice of prior treatment involved regimens known not to result in a sustained suppression of serum testosterone to castrate levels (e.g., monotherapy with nonsteroidal antiandrogens, low-dose estrogens, or 5α-reductase inhibitors). Figure 110–1 Prostate cancer clinical states. PSA, prostate-specific antigen. (Modified from Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148–59.) Table 110–1 Metastatic Castration-Resistant Prostate Cancer: Survival According to Mode of Progression Data from Eisenberger M, Crawford E, McLeod D, et al. The prognostic significance of prostate specific antigen in stage D2 prostate cancer: interim evaluation of Intergroup 0105. Proc Am Soc Clin Oncol 1995;14:abstract 613. For several years it has been postulated that discontinuation of androgen suppression in nonorchiectomized patients may adversely influence the outcome of disease in terms of progression and survival (Taylor et al, 1993). Similarly, it has been shown that administration of exogenous testosterone and its derivatives may indeed produce a significant clinical flare that results in severe pain and neurologic, urologic, and coagulation complications in a small proportion of patients (Fowler and Whitmore, 1981; Manni et al, 1988). In a retrospective analysis of 205 patients with castration-resistant disease treated with chemotherapy, various prognostic variables including orchiectomy were evaluated by Hussain and associates (1994). A multivariate analysis failed to indicate a significant correlation between prior orchiectomy and improved time to disease progression and survival. In these patients all medical forms of androgen deprivation were discontinued at least 4 weeks before initiation of chemotherapy and, contrary to that suggested by Taylor and colleagues (1993), this did not significantly affect outcomes. Until this issue is resolved the general consensus is to maintain all patients on luteinizing hormone–releasing hormone (LH-RH) agonists indefinitely, even during the course of chemotherapy. Another important management aspect relates to antiandrogen withdrawal effects (Scher and Kelly, 1993; Small and Srinivas, 1995; Small et al, 2004). Discontinuation of antiandrogens (both steroidal and nonsteroidal) can result in short-term clinical responses expressed by decreases in PSA levels, symptomatic benefits, and, less frequently, objective improvements in soft tissue and bone metastasis in a small proportion of patients. Because of this, it has been recommended that in patients treated with antiandrogens in combination with other forms of androgen deprivation (e.g., LH-RH agonists) the first step should involve the discontinuation of these agents and careful observation including serial monitoring of PSA levels for a period of 4 to 8 weeks before embarking on the next therapeutic maneuver. The next step is to determine which modality of treatment should be employed first, either administration of second-line hormonal manipulation or cytotoxic chemotherapy. There is an increasing body of data on second-line endocrine therapies suggesting that there may be a role for this approach before institution of chemotherapy (Small and Vogelzang, 1997; Small et al, 2004; Ryan 2006; Ang et al, 2009). Although initial response rates range between 20% and 60%, the median duration of such responses is short ranging, between 2 and 4 months. Agents that have been reported to produce some benefit in this setting include diethylstilbestrol (Smith et al, 1986), aminoglutethimide (Sartor and Myers, 1995), ketoconazole (Small et al, 2004), as well as corticosteroids (Storlie et al, 1995). In view of the potential higher toxicity profile associated with cytotoxic chemotherapy, a sequential hormonal approach may be a reasonable alternative for those patients with relatively limited metastatic disease who remain asymptomatic at the time of disease progression (e.g., rising serum PSA value without other clinical manifestations). Another important consideration is the initial clinical assessment of the potential biologic behavior of these tumors. Evolving data evaluating the role of PSA dynamics suggest that the PSA doubling time (PSADT) predicts for the rapidity of bone scintiscan progression and survival (D’Amico et al, 2005; Armstrong et al, 2007; Robinson et al, 2008). Patients with PSADTs shorter than 3 months have a particularly rapid clinical course and should be considered for more aggressive management approaches. In addition, poorly differentiated and anaplastic/neuroendocrine tumors usually have a low likelihood of significant and durable responses to androgen deprivation. The anaplastic/neuroendocrine phenotype is rare and requires special therapeutic considerations. It has been suggested that systematic biopsies of disease sites in patients with clinically aggressive disease and relatively low serum PSA levels may demonstrate evidence of a neuroendocrine component by immunostaining, which may be of prognostic and therapeutic significance (see later). The usefulness of systematic biopsies in all patients with extensive metastasis and relatively low PSA levels, however, needs to be better defined before routine clinical application. A number of clinical trials using second-line hormonal manipulations and noncytotoxic interventions (bone-targeted treatments) focusing on time to development of bone metastasis are providing some useful information. A report of 201 patients from a prospective clinical trial comparing the effects of the bisphosphonate zoledronate and placebo in biochemically progressing (M0), castrate-resistant disease suggests that the time to bone scintiscan positivity may be very long. At 2 years, only 33% of these patients had evidence of bone metastasis, with a median time to bone metastasis in this group of 30 months. The baseline PSA level (>10 ng/mL) and the PSA velocity independently predicted time to bone metastasis and survival (Smith et al, 2005). In a retrospective review of a similar group of patients prescribed ADT before the development of metastatic disease the median time to clinical metastasis was 9 months. The pretreatment PSA level and the PSA nadir on ADT predicted this outcome (Dotan et al, 2005). The wide difference observed with these two reports (30 months vs. 9 months for time to bone scan metastasis) underscores the heterogeneity of this group of patients and the need for careful prospective evaluation. Their outcome is dependent on various factors, among which are pre-ADT characteristics (pretreatment PSA level, PSADT, initial stage, Gleason score), as well as response to hormonal treatment. The rate of progression may be estimated by PSA constructs such as PSADT and PSA velocity. Patients with metastatic CRPC represent a heterogeneous group with respect to their clinical status at the time of disease progression (Fig. 110–2). Metastatic adenocarcinoma of the prostate has an overwhelming predilection to involve the bone. Although the explanation for this unique metastatic pattern has not been completely elucidated it probably reflects the combination of various biologic factors starting at the time of metastatic spread. Circulating prostatic adenocarcinoma cells are arrested in the cortical and medullary bone spaces, where they subsequently adhere to bone surfaces through specific receptors for moieties such as integrins, collagens, laminin, and other bone-derived proteins. Cell growth is subsequently promoted by various factors such as hormones, growth factors, and stromal-epithelial biologic interactions, most of which operate in the bone marrow. Expansion of tumor from the bone may cause pain, compression, or pathologic fractures, and extensive bone marrow replacement may cause impairment in hematologic function. Clinical involvement of visceral sites is relatively uncommon even in patients with widespread castration-resistant disease. Table 110–2 illustrates the distribution of metastatic sites reported in selected chemotherapy phase 2 and phase 3 trials. These figures suggest that clinical evidence of visceral metastasis is observed in less than 10% of patients, whereas about 20% have demonstrable soft tissue nodal disease. Because the majority of tumor burden in metastatic prostate cancer is found in bone, responses to treatment (e.g., tumor shrinkage) in soft tissue sites alone (e.g., nodal or visceral sites) may not reflect a major treatment benefit because it represents only a small proportion of the overall disease burden. Table 110–2 Typical Distribution of Metastatic Sites in Patients with Castration-Resistant Prostate Cancer Entered in Clinical Trials Patients with metastatic CRPC may present with a range of hematologic problems caused primarily by the disease or by its treatment. Anemia is the most common hematologic abnormality, which can be explained by a variety of factors, such as anemia of chronic disease, bone marrow invasion, blood loss, and rarely secondary to a microangiopathic hemolytic anemia usually associated with a consumption coagulopathy (disseminated intravascular coagulation). A decrease in the red blood cell count of patients with advanced CRPC commonly results from a combination of factors, such as prior treatment with local irradiation of bone marrow, systemic use of radiopharmaceuticals, long-term androgen deprivation, and systemic chemotherapy, as well as from extensive bone marrow invasion by tumor resulting in substantial decrease in bone marrow reserves. The use of erythropoietin can be effective, especially in patients with a history of long-term androgen suppression, and repeated administration may require the use of iron preparations. However, erythropoietin-stimulating agents should be used with caution, because evidence is now emerging that these agents may increase mortality in cancer patients (Bennett et al, 2008). Granulocytopenia and thrombocytopenia are most commonly a complication of extensive radiation therapy or systemic chemotherapy. Rarely, rapidly growing tumors with bone marrow involvement result in pancytopenia. Thrombocytosis is also a nonspecific manifestation associated with various neoplastic conditions, including prostate cancer. Clotting complications associated with thrombocytosis are rarely seen in patients with prostate cancer, and treatment is not usually necessary. One of the greatest emergencies in oncology is the development of epidural spinal cord compression (Sorensen et al, 1990). Because of the frequent involvement of vertebral bodies by metastatic prostate cancer, the incidence of cord compression is of particular concern (see later). Key Points: Clinical Considerations The evaluation of chemotherapy based on uncontrolled clinical trials in patients with metastatic prostate cancer is usually confounded by significant methodologic challenges. The most common metastatic site is bone (nonmeasurable disease), manifested by diffuse osteoblastic lesions that cannot be measured reliably by current methods to allow assessments of therapeutic benefits. Soft tissue or visceral metastatic sites (measurable disease) that allow serial measurements are uncommon (see Table 110–2) and represent only a small fraction of the total burden of the disease. Selection of bi-dimensionally measurable disease sites to assess therapeutic efficacy with serial “tumor measurements” has been the subject of significant criticism. Patients with soft tissue metastasis (especially visceral disease) are considered by many a subgroup with biologic and clinical features distinct from those of the usual patient with prostate cancer who presents with bone metastasis only. A number of prognostic models evaluating baseline and post-treatment characteristics have been developed (Smaletz et al, 2002; Halabi et al, 2003; Armstrong et al, 2007). Among various clinical and laboratory parameters with consistent prognostic significance are baseline functional status (performance status), presence of pain, and pretreatment hemoglobin levels (cutoffs range from 10 to 12 g/dL). Other possible parameters are the baseline PSA level, extent of bone scintiscan involvement (number of lesions or pattern/distribution of bone involvement), and presence of visceral disease. Semi-quantitative methods to evaluate PSA mRNA expression in circulating cells (using reverse transcriptase/polymerase chain reaction) and various PSA constructs (e.g., PSADT and PSA velocity) are among the post-treatment parameters most likely to be of prognostic significance (Kantoff et al, 2001; Scher et al, 2004; Armstrong et al, 2007). Preclinical observations have suggested that several drugs may reduce PSA secretion without affecting tumor growth (Larocca et al, 1991; Eisenberger and Nelson, 1996; Seckin et al, 1996). Whereas these laboratory observations are likely to be clinically relevant, assays used to evaluate a separate drug effect on PSA secretion still require careful validation. A PSA consensus meeting developed by several leading investigators in the field generated initial guidelines with regard to the use of the PSA test for clinical trials in patients with CRPC (Bubley et al, 1999). These guidelines were recently updated and now also provide a consensus on the use of radiologic end points as well as clinical end points (e.g., pain) for the evaluation of men with CRPC (Scher et al, 2008). The use of serum acid phosphatase and alkaline phosphatase has not been proven beneficial. Undoubtedly, new biomarkers are needed to enhance our ability to rapidly identify active treatments for CRPC. Evolving noncytotoxic and targeted therapies may require a new set of end points and identification of drug-specific intermediate biomarkers that reflect mechanism-specific biologic activity. Most of the chemotherapeutic agents available in oncologic practice have been employed in patients with CRPC, either as single agents or in various combinations. Examples have included cyclophosphamide, 5-fluorouracil, estramustine, vinorelbine, etoposide, cisplatin, carboplatin, doxorubicin, mitoxantrone, paclitaxel, and docetaxel (Eisenberger, 1988). With the exception of docetaxel and perhaps mitoxantrone, most other cytotoxic agents are no longer being used with frequency because they have not been associated with either symptomatic improvements or extension of survival. Evolving data with selected chemotherapy during the new millennium suggest that the survival of patients with advanced CRPC is now somewhere between 16 and 18 months (Petrylak et al, 2004; Tannock et al, 2004) as opposed to 6 to 12 months as previously described (Eisenberger, 1988). A first step forward in the chemotherapeutic management of CRPC came with mitoxantrone. This agent, a semi-synthetic anthracycline, had previously shown modest symptomatic benefits but with minimal evidence of objective antitumor activity (Osborne et al, 1983; Rearden et al, 1995). In addition, mitoxantrone appeared to have its maximal palliative effect in combination with low-dose corticosteroids (Moore et al, 1994). In two seminal prospective randomized trials of mitoxantrone plus prednisone versus prednisone alone (Tannock et al, 1996) or mitoxantrone plus hydrocortisone versus hydrocortisone alone (Kantoff et al, 1999), the combination resulted in significant improvements of various quality of life parameters, including pain, but survival was not significantly improved in either trial. These studies provided the justification for the approval in 1997 by the U.S. Food and Drug Administration (FDA) of the combination of mitoxantrone and prednisone for symptomatic metastatic prostate cancer. The next significant advance in the use of chemotherapy for CRPC came with docetaxel, a member of the taxane family. This agent acts by inducing apoptosis in cancer cells through TP53-independent mechanisms that are thought to be due to inhibition of microtubule depolymerization and blockade of antiapoptotic signaling. The induction of microtubule stabilization intracellularly through β-tubulin interactions causes guanosine triphosphate (GTP)-independent polymerization and cell cycle arrest at G2M. In addition, docetaxel has been found to induce BCL2 phosphorylation in vitro, a process that has been correlated with caspase-3 activation and loss of its normal antiapoptotic activity. Unable to inhibit the pro-apoptotic molecule BAX, phosphorylated BCL2 may also induce apoptosis through this alternate pathway. However, additional mechanisms may also be important, such as CDKN1B (formerly p27) induction and repression of BCL-XL. Data with docetaxel monotherapy previously suggested that this compound has significant activity as a single agent (Friedland et al, 1999; Picus and Schultz, 1999; Beer, 2004). As of 2004, docetaxel has become the agent of choice for treatment of metastatic CRPC on the basis of a large phase 3 randomized trial, TAX 327 (Figs. 110-3 to 110-5 and Tables 110-3 to 110-5), which demonstrated superiority over the previous standard, mitoxantrone and prednisone (Tannock et al, 2004). TAX 327 enrolled 1006 patients with no prior chemotherapy and stable pain scores to one of three study arms (all with concomitant prednisone at 5 mg orally twice daily): mitoxantrone, 12 mg/m2 intravenously every 21 days; docetaxel, 75 mg/m2 intravenously every 21 days; or docetaxel, 30 mg/m2 intravenously every 7 days. Patients remained on gonadal suppression (e.g., LH-RH agonists) but had all other hormonal agents discontinued. Treatment duration was 30 weeks in all study arms, or a maximum of 10 cycles in the every-3-week study arms, with more patients completing treatment in the every-3-week docetaxel group than in the mitoxantrone group, mostly because of differences in disease progression (46% vs. 25%). After a median follow-up of 20.7 months, overall survival in the every-3-week docetaxel group was 18.9 months (with a pain response rate of 35% and a PSA response of 45%), contrasted to weekly docetaxel at 17.3 months (and 31% and 48%), respectively. This translated into a 24% relative reduction in the risk of death (95% confidence interval [CI], 6% to 48%; P = .0005) with 3-weekly docetaxel (see Fig. 110–4). Patients on the mitoxantrone arm had a median survival of 16.4 months, a pain response of 22%, and a PSA response of 32%. Figure 110–5 Survival in subgroups, docetaxel every 3 weeks versus mitoxantrone. KPS, Karnofsky performance status score. Toxicity in the 3-weekly versus weekly docetaxel groups was notable for more hematologic toxicity in the every-3-week group (3% neutropenic fever vs. 0%; 32% grade-3/4 neutropenia versus 1.5%) (see Table 110–5) but slightly lower rates of nausea and vomiting, fatigue, nail changes, hyperlacrimation, and diarrhea. Neuropathy was slightly more common in the every-3-week group (grade 3/4 in 1.8% vs. 0.9% in the weekly treated group). Quality of life responses as measured by the FACT-P scores did not differ significantly among the docetaxel schedules but were more favorable than in the mitoxantrone group. The Southwest Oncology Group (SWOG) 9916 study was a second large phase 3 trial (Figs. 110-6 to 110-8) (Petrylak et al, 2004) in which 770 patients with progressive CRPC were randomly assigned to oral estramustine (280 mg three times daily) plus docetaxel (60 mg/m2 every 21 days) versus mitoxantrone (12 mg/m2 every 21 days) plus prednisone. In this study, median overall survival was longer in the docetaxel-estramustine group than in the mitoxantrone-prednisone group (17.5 vs. 15.6 months, P = .02), with a corresponding hazard ratio (HR) for death of 0.80 (95% CI, 0.67 to 0.97). Because of the high rate of thromboembolic events with estramustine, prophylactic low-dose warfarin and aspirin were added to that study arm, which did not reduce the incidence of thromboembolism. Similarly, 20% and 15%, respectively, of patients in the docetaxel-estramustine arm had grade 3/4 gastrointestinal and cardiovascular toxicities. Although comparisons between the docetaxel arms across these two seminal trials may not be appropriate because of differences in schedule, patient populations, and docetaxel dosing (60 mg/m2 in SWOG 9916 and 75 mg/m2 in TAX 327), it may be concluded that estramustine is unlikely to add significantly to the activity of single-agent docetaxel. Figure 110–6 SWOG 9916 study design. D/E, docetaxel plus estramustine; M/P, mitoxantrone plus prednisone. Figure 110–7 Overall survival, SWOG 9916. D+E, docetaxel plus estramustine; M+P, mitoxantrone plus prednisone. Until recently, effective life-prolonging therapies for men with docetaxel-refractory prostate cancer were lacking. This changed in 2010, when the FDA approved a second chemotherapy agent, cabazitaxel, for the treatment of metastatic CRPC. Cabazitaxel (Jevtana, Sanofi-aventis, Paris, France) is a novel tubulin-binding taxane that differs from docetaxel and paclitaxel due to its poor affinity for P-glycoprotein, the ATP-dependent drug efflux pump (Paller and Antonarakis, 2011). In preclinical studies using cancer cell lines and mouse xenograft models, cabazitaxel was shown to be active in both docetaxel-sensitive tumors as well as those with primary or acquired docetaxel resistance (Attard et al, 2006). The first hint of cabazitaxel’s safety and efficacy in men with prostate cancer came during phase I testing, where cabazitaxel was administered by intravenous infusion every 3 weeks at escalating doses of 10 to 25 mg/m2 (Mita et al, 2009). In that study, the principal dose-limiting toxicity was neutropenia. Given the lack of cross-resistance to this agent with docetaxel, and early reports of responses in men with CRPC from this phase 1 trial, a phase 3 trial was launched to evaluate efficacy. The safety and efficacy of cabazitaxel in patients with advanced prostate cancer was definitively evaluated in a pivotal randomized phase III trial (TROPIC) conducted in 146 institutions across 26 countries, and recruited 755 men with metastatic CRPC who had progressed during/after docetaxel-based chemotherapy (de Bono et al, 2010). Of these, 377 patients were randomized to receive mitoxantrone 12 mg/m2 intravenously every 3 weeks (with oral prednisone 10 mg daily), and 378 patients were assigned to receive cabazitaxel 25 mg/m2 intravenously every 3 weeks (plus prednisone). This study was the basis of the FDA’s approval of cabazitaxel with prednisone for the second-line treatment of docetaxel-refractory metastatic CRPC in June 2010. After a median follow-up of 12.8 months, overall survival in men receiving cabazitaxel was 15.1 months compared to 12.7 months in men receiving mitoxantrone (HR, 0.70; P < .0001) (de Bono et al, 2010). Compared to mitoxantrone, cabazitaxel also significantly lengthened progression-free survival (2.8 months vs. 1.4 months; P < .0001), extended time to PSA progression (6.4 months vs. 3.1 months; P = .001), increased radiographic tumor response rates (14.4% vs. 4.4%; P = .0005), and increased PSA response rates (39.2% vs. 17.8%; P = .0002). There were no differences between the two treatment arms with respect to pain responses, or time to pain progression. In subset analyses, the survival advantage of cabazitaxel persisted regardless of whether patients had measurable disease or pain, or whether progression had occurred while receiving docetaxel or following a treatment holiday. In addition, cabazitaxel’s survival benefit was most pronounced for men with ECOG performance status 0-1 (vs. 2), and for patients with disease progression within <3 months of docetaxel initiation (vs. ≥3 months of docetaxel initiation) (de Bono et al, 2010). The last observation implies that cabazitaxel may be effective even in men with truly docetaxel-refractory disease. The most common serious adverse events related to cabazitaxel were hematological, including grade ≥3 neutropenia in 82% of patients (febrile neutropenia in 8%) (de Bono et al, 2010). This degree of myelosuppression begs the question of whether a lower dose of cabazitaxel (e.g., 20 mg/m2) may have been more appropriate, and a randomized trial comparing the safety and efficacy of these two doses (25 mg/m2 vs. 20 mg/m2) is now being conducted. Use of growth factor support should be strongly considered, as reflected in several national guidelines (Mohler et al, 2010). Other nonhematologic toxicities included grade ≥3 diarrhea (6%) and grade ≥3 fatigue (5%). Encouragingly, although peripheral neuropathy (all grades) was observed in 14% of patients receiving cabazitaxel, only 1% developed grade 3 neuropathy. Laboratory and clinical evidence indicates that alterations in the differentiation pathway of prostate cancer can be seen in a small proportion of patients with advanced disease, giving rise to a neuroendocrine/anaplastic transformation (diSant’Agnese, 1995; Nelson et al, 2007). The therapeutic implications of this finding are of significance because tumors demonstrating this phenotype usually represent an inherently endocrine-resistant subtype and, in view of their different clinical and biologic properties compared with the usual adenocarcinoma of the prostate, these tumors usually require different treatment strategies. Such tumors express a number of biologic characteristics unique to neuroendocrine tumors that can also arise from other organs, most commonly the lung. Among these are the expression of receptors to various neuroendocrine peptide growth factors, such as somatostatin, chromogranin A, and serotonin, as well as parathyroid hormone–related protein (PTHrP) and TP53 mutations. These tumors have an uncharacteristic clinical behavior (compared with the usual metastatic prostate cancer), reflected by frequent visceral involvement and rapidly growing soft tissue metastases. Patients frequently present with subacute and often dramatic changes in their disease pattern characterized primarily by a rapidly growing soft tissue mass (frequently involving the primary site but also with retroperitoneal masses), rapid development of visceral (lung and liver) infiltration, osteolytic (as opposed to osteoblastic) bone metastasis, and a high incidence of parenchymal brain involvement (Fig. 110–9). Histologic evaluation of areas demonstrating rapid growth is strongly encouraged. This frequently culminates with demonstration of a small cell variant or a poorly differentiated neoplasm on pathology and the presence of neuroendocrine markers on immunostaining (diSant’Agnese, 1995; Nelson et al, 2007). Interestingly, patients with this tumor phenotype either stop expressing PSA in the presence of major tumor progression or even have undetectable PSA levels at the time of this transformation. Treatment is usually similar to that of patients with other neuroendocrine tumors (e.g., small cell carcinoma of the lung) and includes combinations of cisplatin (or carboplatin) and etoposide (Frank, 1995), paclitaxel, docetaxel, and topotecan. Doxorubicin-containing combinations have been reported to be moderately effective by one group (Papandreou et al, 2002). Radiation therapy is effective and should be considered in cases with bulky disease, with brain metastasis, or when local disease control in critical areas may have a positive impact on quality of life (pain, potential pathologic fractures, and bladder outlet obstruction). A combined chemotherapy and radiation therapy approach is frequently necessary to accomplish maximal disease control. Despite high initial response rates with chemotherapy and radiation treatment, the prognosis of these patients remains poor and is dependent on various factors, including extent and location of metastases. Key Points: The Neuroendocrine/Anaplastic Subtype
Clinical Considerations
Disease Assessment and Treatment Selection
MODE OF PROGRESSION
MINIMAL DISEASE, MEDIAN SURVIVAL
EXTENSIVE DISEASE, MEDIAN SURVIVAL
PSA only
40 months
18 months
PSA + bone scintiscan
23 months
11 months
Nonmetastatic Castration-Resistant Disease
Metastatic Castration-Resistant Disease
Cytotoxic Chemotherapy
Evaluation of Treatment Efficacy
Clinical Trials
The Neuroendocrine/Anaplastic Subtype
Treatment of Castration-Resistant Prostate Cancer
• Rapidly growing disease with the following clinical characteristics should prompt evaluation for the neuroendocrine/anaplastic phenotype: pelvic masses, visceral involvement, osteolytic metastasis with hypercalcemia (associated with high serum PTHrP), and brain metastasis.