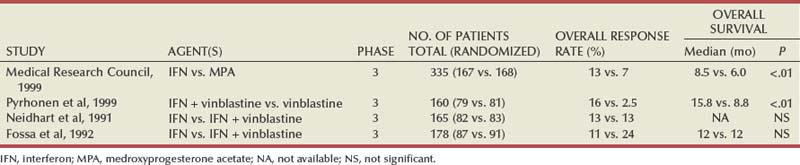
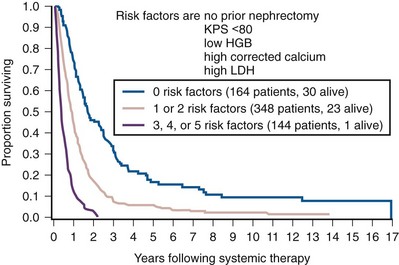
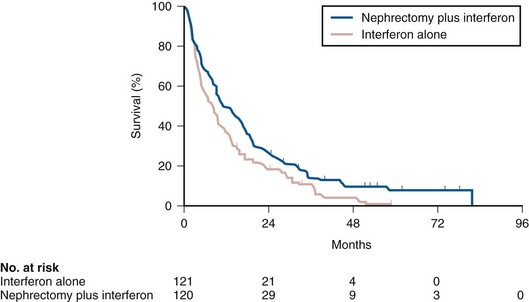
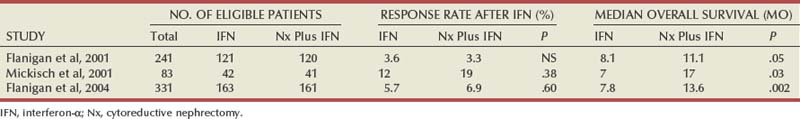
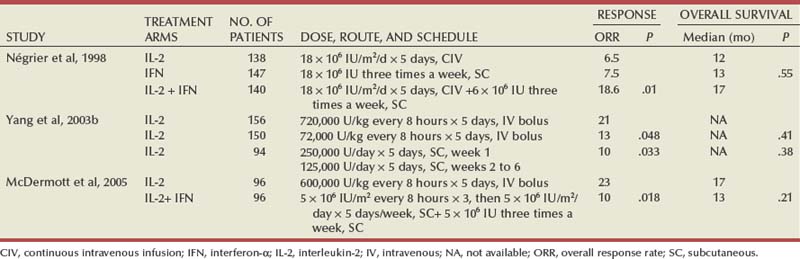
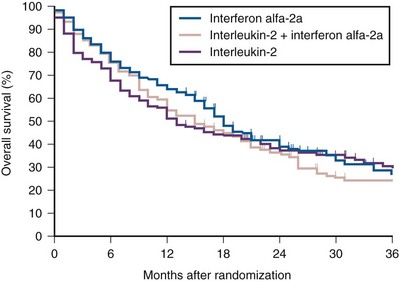
Ramaprasad Srinivasan, MD, PhD, W. Marston Linehan, MD Renal cell carcinoma (RCC) is a term that includes a variety of cancers arising in the kidney and comprises several histologically, biologically, and clinically distinct entities (Linehan et al, 2007, 2009). An estimated 58,240 new cases of cancer arising in the kidney or renal pelvis were diagnosed in 2010 in the United States (Jemal et al, 2010). Approximately one third of all newly diagnosed RCC patients present with synchronous metastatic disease and an additional 20% to 40% of patients with clinically localized disease at diagnosis will eventually develop metastases (Skinner et al, 1971; Rabinovitch et al, 1994; Bukowski, 1997). Metastatic RCC is almost always fatal, with 10-year survival rates of less than 5% (Bukowski, 1997; Motzer et al, 1999, 2000; Motzer and Russo, 2000; Négrier et al, 2002); patients with metastatic disease account for the majority of deaths (approximately 13,000 a year in the United States) related to RCC (Jemal et al, 2010). Advances in our understanding of the genetic and molecular defects underlying the individual subtypes of RCC have led to the development of novel agents designed to reverse or modulate aberrant pathways contributing to renal oncogenesis. These “targeted” therapeutic strategies have largely supplanted other treatment modalities in the initial management of metastatic clear cell kidney cancer; however, surgery, irradiation, and cytokine therapy remain appropriate choices in the management of selected patients with advanced clear cell RCC. Although agents effective in clear cell RCC are often used in patients with other subtypes of RCC there is scant evidence from prospective studies demonstrating benefit in non–clear cell RCC variants. More recently, elucidation of aberrant oncogenic pathways in papillary, chromophobe, and other variants of RCC has paved the way for evaluation of targeted therapeutic approaches in these histologic subtypes (Linehan et al, 2009). Patients with metastatic RCC generally have a poor prognosis, with the majority succumbing to their disease. Ten-year survival in patients diagnosed with metastatic disease was estimated to be less than 5% in the era of cytokine therapy and is unlikely to change significantly with the advent of targeted therapy. However, several clinical features such as a long time interval between initial diagnosis and appearance of metastatic disease and presence of fewer sites of metastatic disease have been observed to be associated with better outcome. Conversely, poor performance status and the presence of lymph node and/or liver metastases are some factors associated with shorter survival. Investigators at the Memorial Sloan-Kettering Cancer Center evaluated a variety of clinical and laboratory parameters in 670 patients enrolled on various clinical trials of chemotherapy or immunotherapy in an effort to identify those pretreatment factors that were able to best predict outcome (Motzer et al, 1999). In a multivariate analysis, a poor performance status (Karnofsky score <80), an elevated serum lactate dehydrogenase (LDH) level (>1.5 times upper limit of normal), a low hemoglobin (less than the lower limit of normal), an elevated corrected calcium concentration (>10 g/dL), and lack of prior nephrectomy were independent predictors of a poor outcome (Table 50–1). Patients could be stratified into three distinct prognostic groups based on these five poor prognostic factors (Table 50–2). The overall survival (OS) times in patients with no adverse factors (favorable-risk group), one to two risk factors (intermediate-risk group), and more than three risk factors (poor-risk group) were 20 months, 10 months, and 4 months, respectively (Fig. 50–1) (Motzer et al, 1999). Subsequently, the same group identified poor performance status, high calcium level, low hemoglobin value, elevated LDH level, and short time interval from initial diagnosis to treatment as factors that could best predict a poor outcome in 463 patients receiving interferon-based therapy in the first-line setting (Motzer et al, 2002). This prognostic model was found to be predictive of survival in an independent data set derived from patients treated at the Cleveland Clinic and provides independent, external validation of the proposed model (Mekhail et al, 2005). Similar prognostic schemes have also been proposed by the Groupe Français d’Immunotherapie and by investigators from the University of California, Los Angeles (Tsui et al, 2000). Validated prognostic models are used in clinical practice to help make appropriate management decisions as well as in the design and interpretation of clinical trials. Modifications of these prognostic schemes as well as identification of reliable molecular markers are under investigation as suitable predictors of response to and survival after therapy with newer targeted agents against vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) pathway components (Choueiri et al, 2007, 2008b; Motzer et al, 2008a). Table 50–1 Adverse Prognostic Factors in 670 Patients Treated with Chemotherapy or Immunotherapy at the Memorial Sloan-Kettering Cancer Center Data from Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40. Table 50–2 Risk Stratification Based on Adverse Prognostic Factors in 670 Patients Treated with Chemotherapy or Immunotherapy at the Memorial Sloan-Kettering Cancer Center Data from Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40. (From Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40.) The role of cytoreductive nephrectomy preceding systemic therapy has been extensively studied in the era of cytokine therapy. The impetus for exploring this approach in metastatic RCC was provided both by the perception that bulky tumors might inhibit key components of the immune system critical for combating cancer and by observations suggesting that removal of large primary tumors may provide clinical benefit. To support this practice early proponents of cytoreductive nephrectomy had cited (1) the rare but well-described occurrence of spontaneous regression of metastatic lesions after nephrectomy (Bloom, 1973; Middleton, 1980; Snow and Schellhammer, 1982; Marcus et al, 1993); (2) preclinical data suggesting that large primary tumors may inhibit T-cell function (Kudoh et al, 1997; Bukowski et al, 1998; Ling et al, 1998; Uzzo et al, 1999a, 1999b); and (3) the inability of systemic agents, particularly cytokines, to induce meaningful responses in primary renal tumors in most patients (Sella et al, 1993; Rackley et al, 1994; Wagner et al, 1999). However, the risk of perioperative morbidity and mortality and the inability of a significant proportion of patients undergoing nephrectomy to subsequently receive systemic therapy clearly underlined the need for unequivocal evidence of clinical benefit as well the ability to identify patients likely to benefit from this approach. Nephrectomy as the sole therapeutic intervention in the context of metastatic disease is unlikely to alter outcome, as suggested by small retrospective analyses (DeKernion et al, 1978). However, several retrospective studies have demonstrated the feasibility of a combined modality approach in which nephrectomy is followed by cytokine therapy, with some suggesting that this approach may favorably impact response and survival. Investigators from the National Cancer Institute (NCI) reported their experience in 195 patients undergoing nephrectomy followed by high-dose interleukin-2 (IL-2) therapy between the years 1985 and 1996 (Walther et al, 1997b). An overall response rate of 18% (including a complete response rate of 4%) after IL-2 therapy was observed in this study. A notable finding that emerged from this study was that although the majority of patients underwent successful resection of the primary tumor only 107/195 (55%) went on to receive IL-2 therapy. Rapid postoperative disease progression and perioperative surgical and medical morbidity were the most common factors preventing delivery of systemic therapy, suggesting that careful patient selection may play an important role in the successful application of this combined modality approach. The impact of patient and/or disease characteristics on outcome is further highlighted by a retrospective study. In Bennett and associates’ (1995) series of 30 patients, which included several patients with unfavorable performance status (Eastern Cooperative Oncology Group [ECOG] 2) and multiple metastatic sites including patients with brain or liver metastases, only 7 (23%) were able to proceed with IL-2 after nephrectomy. Conversely, in a series that included only patients with favorable clinical/prognostic factors (e.g., good performance status, minimal comorbidity, no liver or brain metastases), the majority of patients were able to proceed to systemic therapy after nephrectomy, with high response rates (35% to 40%) and OS (median 20 to 22 months) after cytokine-based treatment (Fallick et al, 1997; Figlin et al, 1997). Although these retrospective analyses and small single-arm prospective studies confirmed the feasibility of a tandem surgical/cytokine therapeutic approach, their major contribution was in laying the foundation for controlled, prospective studies to determine if outcomes with cytokine therapy could be improved by prior nephrectomy. The most compelling evidence in support of cytoreductive nephrectomy is provided by two randomized phase III studies conducted by the Southwest Oncology Group (SWOG) and the European Organization for Research and Treatment of Cancer (EORTC). The larger of the two studies, SWOG trial 8949, randomized 241 patients with metastatic RCC to receive interferon-α-2b either as initial therapy or after cytoreductive nephrectomy (Flanigan et al, 2001). Salient eligibility criteria included a histologic diagnosis of kidney cancer (all histologic subtypes were allowed); good performance status (ECOG 0 or 1); presence of a resectable primary renal tumor; no prior chemotherapy, irradiation, or immunotherapy; and adequate organ function. Although there were no significant differences in the response rates to interferon observed in the two study arms, OS was improved in the surgery plus interferon arm (median 11.1 vs. 8.1 months for interferon alone, P = .05) (Fig. 50–2). These data were similar to those from a smaller EORTC trial (a total of 85 patients randomized to interferon alone or interferon after nephrectomy) that used a similar design and reported a survival advantage favoring the surgery plus interferon arm (median OS 17 vs. 7 months, P = .03) (Mickisch et al, 2001). A combined analysis of both trials revealed data that were consistent with those reported in the individual trials (Flanigan et al, 2004). These data support the use of cytoreductive nephrectomy in carefully selected patients with metastatic RCC who are likely candidates for subsequent cytokine therapy (data summarized in Table 50–3). (From Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 2001;345:1655–59.) Table 50–3 Summary of Outcome in Randomized Studies of Interferon-α Alone or Interferon-α after Cytoreductive Nephrectomy in Patients with Metastatic Kidney Cancer Most studies detailing outcome after metastasectomy are retrospective series. In most series, isolated pulmonary metastases were the lesions most commonly amenable to resection with curative intent. The OS of patients undergoing complete resection of limited metastatic disease is quite impressive, with reported median 5-year survival rates of 35% to 50% in many series (Middleton, 1967; Skinner et al, 1971; Tolia and Whitmore, 1975; O’Dea et al, 1978; Pogrebniak et al, 1992; Kierney et al, 1994; Friedel et al, 1999; Murthy et al, 2005; Russo et al, 2007). The larger of these studies have also attempted to identify patients most likely to benefit from this approach. In a series of 278 patients with recurrent RCC treated at the Memorial Sloan-Kettering Cancer Center, 211 were reported to have undergone either complete (141 patients) or incomplete (70 patients) resection of recurrent tumor from a variety of metastatic sites (Kavolius et al, 1998). In this series, complete or “curative” resection was associated with a longer OS (44% 5-year survival vs. 14% in patients undergoing incomplete resection); multivariate analysis also identified the presence of a solitary metastatic lesion, age younger than 60 years, and a disease-free interval of more than 1 year as favorable prognostic indicators. In addition, some studies have suggested that pulmonary metastases, smaller tumor size (<4 cm in one series), and metachronous lesions are predictors of better outcome after metastasectomy (Friedel et al, 1999; Piltz et al, 2002; Murthy et al, 2005). Although not supported by convincing evidence of survival benefit from prospective studies, resection of isolated metastatic lesions is a reasonable and widely employed practice in selected RCC patients. Cytoreductive nephrectomy can be performed with palliative intent in patients with intractable pain, hematuria, constitutional symptoms, or a variety of paraneoplastic manifestations such as hypercalcemia, erythrocytosis, secondary thrombocytosis, or hypertension. Symptoms such as pain and laboratory abnormalities including hypercalcemia can often be effectively managed medically, whereas symptoms such as hematuria may be amenable to alternative treatment approaches (e.g., angioembolization). Furthermore, resection of the primary renal tumor does not always result in clinical benefit; for instance, in one series, only a little over half the patients (7/12) with hypercalcemia experienced clinically meaningful reductions in serum calcium levels (Walther et al, 1997a). Cytoreductive nephrectomy with palliative intent is therefore performed relatively infrequently but is appropriate in some patients. Resection of metastases to alleviate pain or to forestall potentially life-threatening or debilitating complications is often indicated in a variety of situations. Patients who may benefit from noncurative resection of metastatic lesions include those with solitary brain metastases, metastatic lesions in weight-bearing bones or joints, or vertebral metastatic lesions with impending spinal cord or radicular compromise (Sundaresan et al, 1986; Kollender et al, 2000; Sheehan et al, 2003). Surgical resection is often combined with radiation and/or systemic therapy in many of the aforementioned situations. Key Points: Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma Key Points: Metastasectomy Key Points: Palliative Surgery in Advanced Renal Cell Carcinoma The host immune system has long been believed to play an important role in the causation and control of renal cell cancer. A report detailing spontaneous regression of metastatic lesions after radical nephrectomy provides perhaps the earliest evidence implicating the immune system in the regulation of kidney cancer. The phenomenon of spontaneous regression, thought to represent T- or B-cell mediated antitumor immunity, has sparked great enthusiasm over the years and generated several reports describing this phenomenon (Braren et al, 1974; Silber et al, 1975; Middleton, 1980; Robson, 1982; Snow and Schellhammer, 1982; Kavoussi et al, 1986; Marcus et al, 1993; Edwards et al, 1996). Although rare (it is estimated that the true incidence of spontaneous regression is less than 1%) and often transient, the presumed immunologic mechanisms underlying this event have nonetheless played an important part in the development of immunotherapeutic approaches in kidney cancer. The presence of immune cells, notably cytotoxic T lymphocytes, in resected tumors and the identification of tumor-associated antigens that can serve as human leukocyte antigen (HLA)-restricted targets on tumor cells for T cell—mediated cytotoxicity have also kindled interest in immune-based strategies in renal cell cancer (Finke et al, 1994; Boon et al, 1997; Ada, 1999; Rosenberg, 1999). Early clinical studies explored the efficacy of agents believed to act as nonspecific stimulators of the host immune system components, such as cytokines, with or without adoptive cellular therapy. More recently, investigators have evaluated a variety of novel approaches including allogeneic immunotherapy, vaccines, and modulators of T-cell function. Most immunotherapy strategies have been directed at clear cell RCC, and the utility of these approaches in non–clear cell variants remains to be explored. The interferons are a group of proteins with diverse biologic functions, including immunomodulatory properties. Interferon-α was one of the earliest cytokines to be evaluated for activity in RCC. Initial trials with interferon utilized leukocyte-derived interferon. The subsequent availability of recombinant interferon-α in the early to mid 1980s allowed investigators to evaluate higher doses of this cytokine in a series of phase 2 trials. Initial trials demonstrated overall response rates of 16% to 26% in patients treated with interferon-α, and several subsequent trials have confirmed the activity of this agent, with response rates generally in the 10% to 15% range (deKernion et al, 1983; Quesada et al 1983, 1985, 1989; Umeda and Niijima, 1986; Muss et al, 1987; Rosenberg et al, 1987; Figlin et al, 1988; Minasian et al, 1993; Motzer et al, 2002). The limited long-term survival data available suggest that durable complete responses with this agent are relatively rare (<2%). A variety of dosing schedules and routes have been evaluated to determine the optimal interferon regimen; no single mode of administration or dosing schedule has so far demonstrated superiority over others (Kirkwood et al, 1985; Umeda and Niijima, 1986; Muss et al, 1987; Minasian et al, 1993). Similarly, the addition of chemotherapy or other cytokines to interferon-α has failed to improve the outcomes seen with single-agent therapy (Rosenberg et al, 1989b; Sella et al, 1992; Ravaud et al, 1994, 1998; Ellerhorst et al, 1997; Tourani et al, 1998, 2003; Dorval et al, 1999; Négrier et al, 2000a, 2000b). Several prospective, randomized trials evaluating the efficacy of interferon-α have demonstrated a modest but statistically significant improvement in outcome after treatment with this agent. A randomized phase III study that assigned 335 patients to receive either interferon-α or medroxyprogesterone demonstrated a higher response rate (14% vs. 2%) and OS (median 8.5 vs. 6 months, hazard ratio 0.72, P = .017) favoring the interferon arm of the study (Medical Research Council Renal Cancer Collaborators, 1999). A second study randomized 160 patients with metastatic RCC to receive vinblastine alone or in combination with interferon-α; a higher response rate (16% vs. 2.5%) and improved OS (median 16 vs. 9 months, P = .0049) with the addition of interferon was observed in this study (Table 50–4) (Pyrhonen et al, 1999). Two additional randomized studies suggested that vinblastine is unlikely to have contributed significantly to the activity of this combination by showing that survival was not improved with the addition of vinblastine to interferon-α (Neidhart et al, 1991; Fossa et al, 1992). Lastly, a meta-analysis of randomized trials of interferon against a variety of agents suggested that interferon-based therapy conferred a survival advantage (Coppin et al, 2005). Despite its relatively modest activity, based on the just described data and relative ease of administration compared with IL-2, interferon was commonly the agent of choice in the initial treatment of metastatic RCC until the advent of VEGF pathway antagonists. Clinical trials in the early 1980s initially identified IL-2 as an active agent in RCC, with some IL-2–based regimens leading to objective response rates in excess of 30% (Rosenberg et al, 1989a, 1993). In a subsequent report detailing 255 patients treated on a series of phase 2 trials at the NCI, a more modest overall response rate of 15% (37/255 patients) was noted (Fyfe et al, 1996). Several trials conducted by the NCI and the Cytokine Working Group as well as meta-analyses of published data have consistently demonstrated response rates in the range of 15% to 20% (Lotze et al, 1986; Rosenberg et al, 1987, 1989a, 1993; Fisher et al, 1988; Dutcher et al, 1997a). More importantly, 7% to 9% of patients receiving high-dose IL-2 are reported to have achieved complete regression of all metastatic tumor, with the majority of complete responders (>60%) demonstrating no evidence of disease recurrence on long-term follow-up (Fisher et al, 1997, 2000; Rosenberg et al, 1998). There have also been reports of long-term disease-free remission in partial responders whose limited disease burden after IL-2 therapy rendered them amenable to resection of localized metastases. High-dose IL-2 was approved by the U.S. Food and Drug Administration (FDA) for the treatment of metastatic kidney cancer in 1992, based largely on its ability to induce durable complete responses in some patients. The initial studies with IL-2 were conducted using an intravenous bolus regimen with doses of 600,000 or 720,000 IU/kg administered every 8 hours as tolerated to a maximum of 15 doses. A major limitation of this dosing regimen is the considerable associated toxicity that has limited its widespread use. Vascular leak syndrome, and the resulting hypotension, third-space fluid retention, respiratory compromise, and multiorgan damage are some of the more problematic concomitants of IL-2 therapy and led to an unacceptably high treatment-related mortality rate (2% to 5%) in early studies with this agent (Rosenberg et al, 1987; Kammula et al, 1998). Subsequently, careful patient selection, intensive monitoring schemes, and early interventions with IV fluids, vasopressors, and antibiotics have served to significantly reduce mortality associated with IL-2 (Kammula et al, 1998). However, the significant morbidity and expense associated with delivering bolus high-dose IL-2 have led several investigators to explore alternative regimens aimed at reducing toxicity without compromising efficacy. Numerous single-arm phase 2 studies have evaluated a variety of alternative regimens, including daily subcutaneous administration and continuous intravenous infusion (Escudier et al, 1994a, 1994b, 1995; Atkins et al, 2001; Négrier et al, 2000b, 2005, 2008). Many of these studies have reported overall response rates of 10% to 30%, suggesting comparable efficacy to high-dose bolus administration based on data from historical controls. However, two randomized studies have demonstrated that, although well tolerated, lower-dose regimens are associated with lower overall response rates, as well with fewer durable, complete responses (Table 50–5) (Yang et al, 2003b; McDermott et al, 2005). Table 50–5 Summary of Results from Selected Randomized Trials of Interleukin-2 in Metastatic Renal Cell Carcinoma Attempts to enhance the efficacy of IL-2 therapy have led investigators to explore combination therapy with other cytokines, cytotoxic chemotherapy, and adoptive cellular immunotherapy. Early experience with combination cytokine therapy was promising, with one study reporting a 31% overall response rate in patients treated with high-dose bolus IL-2 and interferon (Rosenberg et al, 1989b). However, subsequent studies have indicated that this combination is more toxic and no more effective than IL-2 alone (Ravaud et al, 1994; Bukowski et al, 1997; Dutcher et al, 1997b; Tourani et al, 1998, 2003). A multicenter, randomized phase III study compared the efficacy of intermediate-dose IL-2 administered by continuous intravenous infusion with interferon-α or the combination in 425 patients with metastatic clear cell RCC (Négrier et al, 1998). Although the combination of IL-2 and interferon resulted in a higher response rate (18.6%) and 1-year event-free survival (EFS 20%) compared with IL-2 (overall response rate 6.5%, 1-year EFS 15%) or interferon (overall response rate 7.5%, 1-year EFS 12%) alone, there was no significant difference in survival between the groups (Fig. 50–3). Combination therapy also resulted in higher toxicity than either agent given alone. Regimens combining IL-2 and cytotoxic chemotherapy (particularly 5-fluorouracil [5-FU]) have been the subject of numerous studies. Unfortunately, reports of high response rates in initial studies (49% in a study using IL-2, interferon, and 5-FU) could not be reproduced in later studies (Atzpodien et al, 1993; Ellerhorst et al, 1997; Dutcher et al, 2000; Négrier et al, 2000a). Similarly, despite the promise of preclinical and early clinical studies, the addition of ex-vivo expanded tumor-infiltrating lymphocytes or lymphokine-activated killer cells to high-dose IL-2 has not reliably demonstrated improved clinical benefit and these approaches have been largely abandoned (Rosenberg et al, 1993; Law et al, 1995; Figlin et al, 1999). (From Négrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Français d’Immunotherapie. N Engl J Med 1998;338:1272–78.) Given the considerable toxicity associated with high dose IL-2 and the relatively small proportion of patients who derive benefit from this therapy, identification of predictors of response and long-term outcome has received considerable attention. The predictive value of a variety of histologic, clinical, laboratory, and molecular parameters has been studied. Patients with clear cell RCC appear most likely to benefit from IL-2 therapy, although the exceedingly small number of patients with non–clear cell histologies typically enrolled in studies of IL-2 makes it difficult to draw definitive conclusions about efficacy in this subgroup of patients. Patient performance status, number of metastatic sites, site of metastases, prior nephrectomy, and time from nephrectomy to systemic therapy are some of the factors that may impact outcome. In one study, patients with more than one site of metastasis, those with metastatic disease within 1 year of diagnosis, and those with liver metastases had the worst outcome, with a median survival of 6 months (Négrier et al, 2002). Overexpression of carbonic anhydrase IX (CAIX or G250) has been observed in a retrospective analysis to be associated with a higher probability of response to IL-2 (Atkins et al, 2005). When combined with histologic features, CAIX expression was found to be predictive of IL-2 responsiveness and OS. The expression of CAIX is regulated by hypoxia-inducible factor (HIF), and both HIF and CAIX are upregulated in tumors with VHL loss; it is therefore unclear if CAIX expression influences the behavior of these tumors or is merely a surrogate for VHL dysfunction. Although intriguing, these data should be validated in prospective trials before CAIX and/or histologic parameters can be reliably used to select patients most likely to benefit from cytokine therapy. Allogeneic hematopoietic stem cell transplantation allows the replacement of host or recipient immune and hematopoietic systems with those of a healthy, HLA-compatible donor. The therapeutic potential of hematopoietic stem cell transplantation lies largely in the ability of the transplanted donor graft to generate an allogeneic antitumor immune response known as the graft-versus-tumor effect. This approach has been used successfully with curative intent in a variety of hematologic malignancies (Thomas et al, 1977; Weiden et al, 1979, 1981). The ability of a variety of immune-based approaches to induce remissions in patients with RCC and evidence suggesting that the host immune system in these patients may be compromised and/or tolerant to tumor cells have led several investigators to evaluate allogeneic hematopoietic stem cell transplantation in kidney cancer. The approach was initially studied by investigators at the National Heart, Lung and Blood Institute exploring the efficacy of reduced intensity hematopoietic stem cell transplantation in patients with treatment-refractory metastatic RCC. Eligible patients underwent reduced intensity conditioning with cyclophosphamide (120 mg/kg) and fludarabine (125 mg/m2) followed by infusion of a granulocyte colony-stimulating factor–mobilized peripheral blood stem cell graft from a 5/6 or 6/6 HLA-matched sibling donor. The initial experience with hematopoietic stem cell transplantation in metastatic RCC was published by Childs and colleagues (2000). Ten of the first 19 patients treated with this transplant approach had tumor shrinkage, including 3 who had a complete response and 7 who had a partial response. As more recently reported, 74 patients have undergone hematopoietic stem cell transplantation for RCC at the National Institutes of Health. Of these, 73 patients have demonstrated durable engraftment, achieving 100% donor T-cell chimerism by day 100 posttransplant. Twenty-nine of 74 (39%) patients have had a disease response, including 7 complete (9%) and 22 partial responders (30%) (Takahashi et al, 2008). Preliminary data suggest that disease response after hematopoietic stem cell transplantation is a clinically meaningful phenomenon because regression of metastatic RCC appears to be associated with a trend toward improved survival. Survival in nonresponders has been less than 6 months, in contrast to those achieving a partial response who survived a median 2.5 years posttransplant. Several durable responses have been noted, and the first patient who underwent a transplant remains in complete remission more than 10 years after the procedure. Hematopoietic stem cell transplantation is associated with a variety of adverse events typically associated with conditioning chemotherapy (e.g., pancytopenia), a variety of opportunistic infections, and graft-versus-host disease (GVHD). Eight patients in the just-mentioned series died of transplant-related causes (transplant-related mortality of 11%), most due to GVHD and its attendant infectious complications. Several other trials have since confirmed the efficacy of this approach in RCC (Table 50–6) (Rini et al, 2001, 2002; Bregni et al, 2002; Artz et al, 2005; Barkholt et al, 2006). However, a Cancer and Leukemia Group B (CALGB) intergroup trial evaluating the feasibility of performing hematopoietic stem cell transplantation for metastatic RCC in a multi-institutional setting in the United States reported no responses in 22 patients undergoing hematopoietic stem cell transplantation from an HLA-matched sibling donor after cyclophosphamide/fludarabine–based conditioning (Rini et al, 2006
Prognostic Factors
RISK GROUP
NO. OF ADVERSE PROGNOSTIC FACTORS
MEDIAN OVERALL SURVIVAL
Good
0
20 months
Intermediate
1-2
10 months
Poor
3-5
4 months
Surgical Management of Metastatic Renal Cell Carcinoma
Debulking or Cytoreductive Nephrectomy in Patients with Metastatic RCC
Resection of Metastases
Palliative Surgery
Immunologic Approaches in the Management of Advanced Clear Cell Renal Cell Carcinoma
Interferons
Interleukin-2
Allogeneic Hematopoietic Stem Cell Transplantation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Treatment of Advanced Renal Cell Carcinoma
• Two randomized studies have demonstrated improved survival in carefully selected metastatic RCC patients undergoing cytoreductive nephrectomy followed by cytokine therapy (interferon-α) compared with those receiving cytokine therapy alone.
• In some patients with advanced RCC, cytoreductive nephrectomy may help alleviate symptoms related to the primary tumor (e.g., intractable pain, hematuria) or paraneoplastic manifestations.