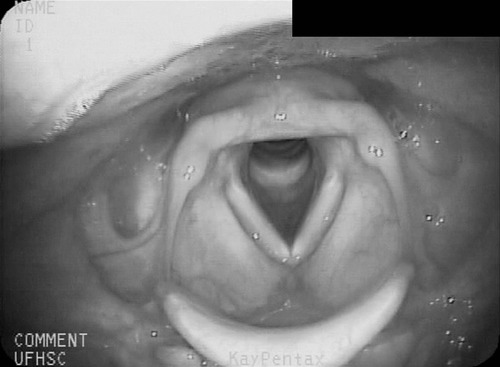
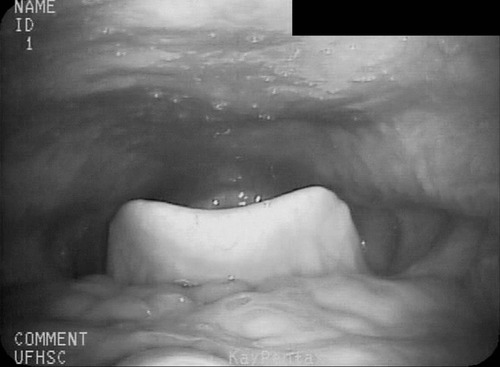
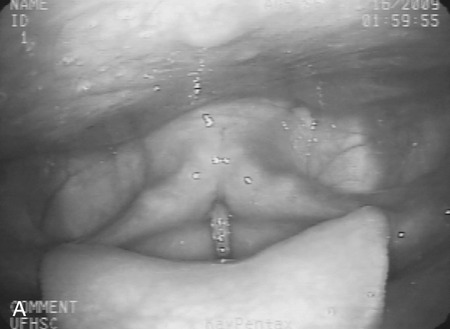
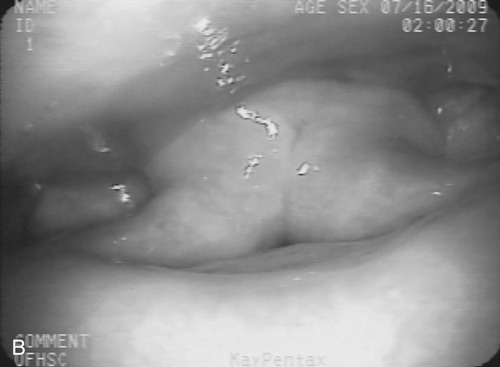
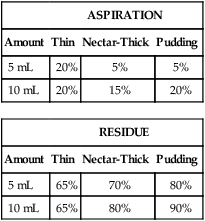
1. Describe some of the basic differences between compensatory techniques and rehabilitative techniques. Tell how this distinction applies to specific therapy techniques. 2. Describe the impact of various therapy techniques on the swallowing mechanism. 3. Identify the expected functional benefits associated with various behavioral treatment strategies. 4. Describe risks from specific therapy techniques that may be posed to patients. 5. Explain strategies that may be helpful in evaluating the appropriateness of existing or novel interventions for a specific patient. As indicated in Chapter 12, practicing clincians should avail themselves of evidence supporting (or refuting) the application of any therapy technique. Beyond evidence, however, clinicians will benefit from a conceptual framework from which any clinical technique might be considered. Following this line of reasoning, some common questions to consider might include (1) “What is the purpose of the technique?” (2) “What are the details of the technique?” and (3) “What is the impact on the swallowing mechanism?” Details of any technique are essential for appropriate clinical application. Even simple techniques may become confused by the clinician and/or the patient. For example, a recent survey1 reported poor agreement in the details of the chin-down posture. This seemingly simple compensation may be more variable than conceived and variants have been described using different terminology. Variability may be clinically important as different postures are likely to have different physiologic and hence functional impact on the swallow mechanism. Thus, a basic question is whether published articles, presentations, or other sources of information provide clear descriptions on how to perform or teach the technique under consideration. This information might incorporate a clear description of the technique and specific instructions for how to apply the technique clinically. This information should include how often and under what conditions the technique should be used. Furthermore, it is important to determine whether the published evidence was gathered from a group of patients similar to the patient being considered for a given technique. For example, evidence supporting the use of a given technique for stroke patients may not be applicable to patients with head/neck cancer. An additional consideration for any therapy technique would be to understand the intended impact on the swallow mechanism. The impact of any technique relates to the outcome of the intervention using that technique. Ultimately, intervention should result in functional benefit for any patient, but what are the specifics of the intended functional benefit? Following terminology from Chapter 12, the technique would be the action plan used by the clinician. Techniques would have expected physiologic impact(s) on the swallow mechanism that are related to the objectives of treatment. If successful, the goal or functional outcome of therapy would be realized. Thus it is important for the clinician to understand how the specific technique relates to the intended physiologic impact on the swallow mechanism and how this change will relate to the functional benefit sought by intervention. Box 14-1 lists common swallow therapy techniques described in dysphagia treatment literature. Each technique has some degree of supporting evidence in subgroups of patients with dysphagia. The following information is presented (if available) for each technique: (1) purpose of the technique, (2) details of the technique, and (3) impact on the swallow mechanism. Postural adjustments may involve the entire body or only the head. In general, changes in head and/or body posture are considered effective in reducing aspiration in various patient groups.2,3 Reported results suggest that change in posture has the potential to redirect the bolus and may change the speed of bolus flow, thus giving the patient more time to adjust the swallow. Generally, changes in body posture are considered compensatory and thus fall under the category of dysphagia management. In some clinical situations, notably in patients who have abnormal postures because of physical reasons, adjustments in body posture to facilitate safe swallowing may be long term. However, even in these situations, adjustments in body posture are considered compensations rather than attempts to change the dynamics of the abnormal swallow. Furthermore, Logemann4 appropriately notes that no single posture improves swallowing function in all patients. Thus, depending on the specific swallowing deficits presented by an individual patient, the treating clinician may use one or more compensatory postures to facilitate safer swallowing function. Beyond posture, clinicians may find it necessary to use additional compensations to facilitate safe swallowing function in some patients.5 Typically, body posture changes involve lying down and/or side-lying. Both changes are expected to reduce the impact of gravity either during the swallow or on post-swallow residue. The side-lying technique may be applied when a difference in pharyngeal function is noted between the right and left sides. In this situation, conventional wisdom suggests that the stronger side be the down side. This position uses gravity to direct the bolus (or residue) toward the stronger hemipharynx. The direct clinical impact of altering body posture on the swallow, specifically on airway protection, may be assessed during the instrumental swallowing examination. One physiologic result of lying down during swallowing may be increased hypopharyngeal pressure on the bolus, contributing to increased maximum opening of the pharyngoesophageal sphincter (PES) and reduced duration of sphincter opening during the swallow.6 These physiologic changes may be helpful in strengthening the swallow in some patients. Postural adjustments may not be the ideal intervention for patients who are at risk for noncompliance due to physical or cognitive limitations. Also, change in body posture, specifically any variant of lying down, may affect esophageal motor functions.7,8 An additional functional consideration may be the impact of supine position on reflux episodes.9 Patients with severe reflux (even those being fed via tube) may benefit from maintaining an upright posture during and after feeding. The upright posture helps reduce or prevent reflux that may contribute to aspiration. In addition, nocturnal head of bed elevation has long been advocated for patients with nocturnal reflux. This simple postural adjustment is highly effective in promoting acid clearance from the esophagus.10 Thus clinicians should evaluate the impact of body posture variations on esophageal functions during the instrumental swallow examination. This guideline especially applies to patients with clinical symptoms or signs of esophageal deficit. Head extension may be accomplished by raising the chin. This has the anatomic effect of widening the oropharynx (Figure 14-1) and may be helpful in moving a bolus from the mouth into the pharynx when oral/lingual deficits are present. Thus patients who have received a glossectomy, other oral resection, reconstruction, or patients who have significant lingual paralysis may benefit from use of a head extension technique. The basic concept is to elevate the chin and use gravity to assist in oral bolus transit toward the pharynx. The patient should be determined to have adequate pharyngeal function and adequate laryngeal closure for airway protection. Clinical benefit (reduced aspiration) from the head extension posture used during the fluoroscopic swallow examination has been demonstrated in a small number of patients treated for oral cancer.2 Conversely, with head extension, defective laryngeal closure was observed in appoximately one third (10/35) of patients demonstrating normal laryngeal closure during swallowing in a head neutral position.11 Head extension may also affect the PES and the coordination between pharyngeal and PES activity. Specifically, head extension increases intraluminal pressure (less relaxation), decreases duration of relaxation in the PES, and changes the temporal coordination between pharyngeal and PES swallow pressures as measured manometrically.12 These changes may complicate an existing swallowing problem. Thus head extension may be a useful clinical technique in patients with difficulty transporting a bolus from the mouth to the pharynx, but it may contribute to swallowing difficulties in patients who have airway protection or PES deficits. Like most compensatory maneuvers, the impact of head extension on swallow function may be evaluated during the instrumental swallow examination. Head flexion has been suggested as a technique to facilitate improved airway protection in patients who demonstrate deficits in airway protection during swallowing.11 Flexing the head (chin tuck) has the anatomic effect of improving laryngeal vestibule closure,11 narrowing the oropharynx13 (Figure 14-2), and reducing the distance between the hyoid bone and the larynx.14 The physiologic effects of head flexion (chin tuck) in patients with dysphagia are reported as minimal. In patients with pharyngeal dysphagia, no manometric differences were found between control swallows and swallows using the chin-tuck maneuver.14,15 Furthermore, in a small sample of healthy volunteers, weaker pharyngeal contractions were observed during swallows using the chin-tuck position.16 Moreover, the combination of a reclining posture (60 degrees) with a chin tuck (60 degrees) may significantly increase the duration of swallowing apnea.17 This change in respiratory pattern may contribute to increased respiratory stress in some patients. At the very least, clinicians should monitor pharyngeal residue and any respiratory changes induced by introduction of the chin tuck or any swallowing maneuver. Clinical benefit from the chin-tuck maneuver has been desribed primarily in reference to improved airway protection. Shanahan et al.18 reported elimination of aspiration with the chin tuck in 15 patients with dysphagia resulting from neurologic damage. These investigators also reported that this postural maneuver was not useful for patients who demonstrated delay in swallow initiation and post-swallow residue in the piriform recesses. From a larger, heterogeneous sample, Rasley et al.3 reported that the chin-tuck position eliminated aspiration on all tested volumes in 21 of 84 (25%) patients. Logemann, Rademaker, and Pauloski2 reported that 5 of 6 (83%) patients with head and neck cancer–related dysphagia were able to eliminate aspiration on at least one bolus volume of liquid barium during the fluoroscopic swallowing study. Lewin et al.19 reported elimination of aspiration for liquids using the chin-tuck position during the fluoroscopic swallow examination in 17 of 21 patients after esophagectomy. Finally, Logemann et al.20 conducted a large randomized study to evaluate the effectiveness of the chin-down posture (chin tuck) compared with thickened liquids in the reduction of aspiration in patients with dysphagia related to dementia or Parkinson’s disease. Results indicated that the chin-down posture was less effective than thickening liquids in reducing aspiration events during the fluoroscopic swallow examination. Note that each of these studies evaluated the impact of the chin-tuck position within the confines of the fluoroscopic swallow examination. Thus each study describes the effect of this posture as a short-term compensation. Zuydam et al.21 reported that compensatory maneuvers (chin tuck and supraglottic swallow) used as therapy techniques were effective in 50% of patients who aspirated. In a companion paper to the effect study of chin tuck versus thickened liquids,19 Robbins et al.22 monitored a subgroup of the original patients for 3 months after the initial swallow examination. Patients were randomly assigned to one of the three interventions (chin tuck for thin liquids, nectar-thick liquids, or honey-thick liquids) as a management strategy and the rate of new pneumonia (incidence) was evaluated as the primary outcome. Results indicated no significant differences in the rates of pneumonia across the three interventions. The chin-tuck position may be helpful in reducing or elminating aspiration in some patients with dysphagia. However, it does not produce benefit in all patients and may be inferior to thickened liquids in some patients. Although anatomic adjustments have been demonstrated in response to this posture, physiologic changes reportedly are minimal. Furthermore, at least one study raises the possibility that this posture, especially combined with a reclining body position, may alter the coordination of swallow and respiration. Finally, it is possible that this technique may need to be combined with other strategies, including other postures or bolus changes, to produce maximum benefit.2 Because this is such a simple task to perform, its impact on appropriate patients should be evaluated during instrumental swallow studies. In this chapter we have used the term chin-tuck to refer to a specific compensatory posture. We have also used terms from published descriptions including head flexion and chin-down posture. This variability in terminology is given a practical focus by the survey results of Okada et al.,1 who remind us that different postures may result in different physiologic or functional results. Thus what may seem simple to clinicians may be confusing to patients. In evaluating research on any technique, clinicians need to look beyond the terminology and be certain of the technique. Furthermore, when evaluating the impact of any technique or instructing patients, clarity and consistency are very important. Head rotation or the head-turn maneuver is another postural adjustment that can function as an effective short-term compensation to improve swallowing function. The head-turn posture has been advocated primarily in cases of unilateral pharyngeal deficit.23,24 Conventional wisdom suggests that patients turn the head toward the weaker side in cases of hemilateral impairment. The anatomic result of this postural maneuver is a narrowing or closing off of the swallowing tract on the side toward which the head is turned. This effect is demonstrated in Figure 14-3 in which the head is turned to each side with the corresponding change in oropharyngeal configuration. However, this closure effect may not extend throughout the hypopharynx but may be restricted to the level of the hyoid bone at the superior hypopharynx, which leaves the inferior aspects of the pharynx open in some patients.25 Physiologic effects of head rotation include a drop in PES pressure and corresponding increase in PES opening.24 Additional physiologic effects of the head-turn position include increased pharyngeal manometric swallow pressures on the side of the pharynx toward which the head is turned, a drop in PES resting pressure opposite the direction of head turn, and a delay in PES closure (e.g., longer relaxation of the PES).26 These physiologic findings suggest that the head rotation technique should be considered for patients with reduced PES opening. The combined anatomic and physiologic changes resulting from turning the head are anticipated to facilitate an increase in the amount swallowed with less residue and reduced risk of airway compromise. Clinical benefit from the head-turn position has been reported in a variety of patient groups. Logemann et al.24 reported improved swallow function (larger amount of bolus swallow/less residue) in all 5 patients (100%) patients with dysphagia after lateral medullary stroke. Within a sample of patients with various causes of dysphagia, Rasley et al.3 reported that liquid aspiration was eliminated for all tested volumes in 20 of 77 (26%) of patients. In a group of postsurgical head and neck cancer patients, Logemann, Rademaker, and Pauloski2 reported 75% effectiveness (9/12 patients) in the elimination of liquid aspiration in at least one volume. Like many postural maneuvers, head rotation should be considered a compensatory technique, not a lifelong adjustment in swallowing. Also, like other techniques in this category, effectiveness may be reduced by compliance, cognitive factors, physical factors, or the presence of multiple swallowing deficits. Moreover, this postural adjustment may be combined with other compensations or maneuvers to improve swallow function.2 Finally, the functional effects of a head-turn maneuver may be checked easily during either the fluoroscopic or endoscopic swallow examinations. Alterations in liquid viscosity (specifically meaning “thickness”) have been advocated in both the evaluation and treatment of patients with dysphagia.27–29 Two major foci seem to emerge in relation to the use of thickened liquids: (1) thicker liquids result in less aspiration among patients with dysphagia and (2) thicker liquids have a physiologic impact on the swallow mechanism. In a 2005 survey of speech-language pathologists experienced in dysphagia intervention,30 the most commonly reported reasons for the use of thickened liquids included delayed onset of swallowing and impaired oral control of thin liquids. Reduction of aspiration was not specifically reported among the most frequent reasons for use of thickened liquids. However, this lack of focus on aspiration seems to be the result of the survey questions, which did not include direct reference to aspiration reduction as a rationale for use of thickened liquids in adult patients with dysphagia. In this survey, the perception of patients’ acceptance of thickened liquids was influenced by the degree of “thickness.” Honey and spoon-thick liquids were considered less accepted (strong dislike) than nectar-thick liquids. Furthermore, these initial negative perceptions either worsened or remained the same with continued use over time. These patterns of patient acceptance also are reflected in the use patterns of thickened liquids among patients in skilled nursing facilities.31 Results of a national review of thickened-liquid application in skilled nursing facilities indicated that approximately 8% of all patients (from a total sample of 25,470) received thickened liquids—60% received nectar-thick liquids, 33% received honey-thick liquids, and 6% received pudding or spoon-thick liquids. Thus thickened liquids are used frequently in the management of adult dysphagia with the most frequent being nectar or syrup consistency. The frequent use of thickened liquids seems to occur in the relative absence of strong evidence that they provide significant clinical benefit to adult patients with dysphagia. In the 2005 survey,30 nearly 85% of responding clinicians indicated that they believed thickening liquids was an effective management compared with only 5% who disagreed with this position. These opinions reflect clinicians’ positive perception, but until recently, only scant empirical support existed for this clinical practice. Kuhlemeier, Palmer, and Rosenberg32 studied bolus factors that influenced aspiration rates among 190 patients with dysphagia and reported that thickness of liquid (thin, thick, ultrathick) and manner of presentation (spoon versus cup) had a direct impact on the rates of aspiration during the fluoroscopic swallowing examination. Ultrathick liquids presented by spoon resulted in the lowest aspiration rates, followed by thick liquids presented by spoon, then by cup with thin liquids resulting in the highest rates of aspiration during the fluoroscopic study. To date, the strongest evidence that liquid viscosity affects aspiration during the fluoroscopic swallowing examination comes from a large randomization trial of techniques to reduce liquid aspiration in patients with dementia or Parkinson’s disease.20 The results of this study support those from the earlier report from Kuhlemeier, Palmer, and Rosenberg.32 Aspiration rates were lowest for honey-thickened liquids (thickest liquid evaluated) and greatest for thin liquids (accompanied by a chin-down posture). Aspiration rates for nectar-thickened liquids were between the two other viscosities and significantly different from both. Interestingly, the reported benefit from honey-thick liquids was not maintained when this viscosity was presented last among the materials examined. The investigators suggested that patient fatigue may have been a factor in this result. Certainly, clinicians should consider patient endurance (converse of fatigue) when interpreting the results of the swallowing evaluation and in making clinical recommendations based on any evaluation. The study by Kuhlemeier, Palmer, and Rosenberg32 implied that manner of bolus presentation may influence the occurrence of aspiration in addition to viscosity. Other bolus characteristics also may affect aspiration rates. For example, in an unpublished study of aspiration and residue rates in adult patients evaluated in the acute care environment, we learned that bolus thickness and volume may interact. Table 14-1 summarizes the rates of aspiration and residue seen during fluoroscopic examination among 20 patients who swallowed 5 versus 10 mL of thin, nectar-thick, and pudding viscosities of barium sulfate contrast agent. Note that different clinical impressions result depending on both the thickness and the volume of the material swallowed. For example, the rates of aspiration and residue are the same for thin liquid across both volumes. However, although thickening material results in reduction or aspiration rates for 5 mL (20% to 5% to 5%), the benefit is not the same for 10 mL (20% to 15% to 20%). The rate of aspiration for 10 mL of pudding is as high as that for 5 or 10 mL of thin liquid. In addition, the rate of residue in the vallecullae increases more for the larger volume as the swallowed material is thickened. This type of pattern implicates the need to evaluate more than just thickness of swallowed material. Therapy and perhaps diet recommendations would differ based on the pattern presented by an individual patient. TABLE 14-1 Potential Interaction Between Viscosity and Volume in Rates* *Percent of aspiration and residue during the fluoroscopic swallowing examination. Although studies such as those reviewed suggest that thickening liquids reduces aspiration rates in groups of patients, clinicians must remember that these are not treatment studies. These studies evaluate the immediate effect of thickening liquids during the fluoroscopic study and do not speak directly to the effectiveness of using thickened liquids as an intervention or longer-term management strategy. In fact, the single clinical trial22 to date indicated no significant differences in pneumonia rates across patients who used thickened liquids versus a chin-down posture to reduce aspiration over a 3-month period. Thus the clinical benefits, especially long-term benefits from continued use of thickened liquids as a management strategy, are unclear. Morever, continued use of thickened liquids may impose other health risks to adult patients with dysphagia. A primary concern is the risk of dehydration from reduced fluid intake. Elderly patients, especially those with dysphagia, are considered at increased risk for dehydration secondary to reduced fluid intake.33 Combining this potential risk with the results of surveys indicating a high rate of dislike of thickened liquids among adult patients with dysphagia suggests that reduced fluid intake, especially of thickened liquids, may further the risk of dehydration in this patient population. In one small randomized clinical trial,34 stroke patients with dysphagia were assigned to receive thickened liquids or thickened liquids plus water. Patients in the combination liquid condition (water plus thickened liquids) ingested less-thickened liquids and had greater daily fluid intake than those in the thickened-liquid–only condition. Neither group experienced significant respiratory complications. Related to this study are the clinical experiences of more than 20 years from the Frazier Rehabilitation Institute.35 The Frazier Water Protocol has never been objectively evaluated, but in more than 20 years of application, this single rehabilitation hospital has allowed patients with dysphagia, including those considered to aspirate thin liquids, access to water between meals. They have experienced impressive outcomes with few instances of dehydration (5/234 or 2.1%) or chest infection (2/234 or 0.9%) among a large sample of patients (N = 234) who followed this protocol over an 18-month period. Despite the absence of more rigorous scientific evaluation, these long-term experiences from the Frazier Rehabilitation Institute provide a compelling argument for clinicians to consider access to water for patients with dysphagia, even those who may aspirate thin liquids. A note of caution, however—the Frazier Water Protocol is more complex that just providing water to patients with dysphagia. Clinicians are advised to review thoroughly the complete protocol before implementing this strategy. Thickening liquids also may affect swallow physiology. For example, increasing liquid viscosity has been shown to increase lingual-palatal contact pressures during swallowing by healthy volunteers.36 Furthermore, increasing liquid viscosity may slow the transit of a bolus37,38 and increase pharyngeal pressure and upper esophageal sphincter relaxation.38,39 These studies and others implicate the tendency of the healthy swallow mechanism to accommodate to different bolus characteristics—in this specific instance, liquid viscosity. However, aside from timing alterations, few studies have evaluated bolus accommodation to varying liquid viscosities in adults with dysphagia, and at least one study has suggested that increasing liquid viscosity did not affect the timing or bolus propulsive force of swallows performed by adults with neurogenic dysphagia.40 Thus differences may exist in bolus accommodation between healthy adults and adults with dysphagia. Given the prevalence of liquid modifications in clinical management, the impact of thickening liquids on swallow physiology in adult patients seems an important area of clinical investigation. In the preceding section bolus volume, viscosity, and method of presentation were introduced as variables that may affect a patient’s performance relative to aspiration of liquids during the fluoroscopic swallowing study. Another liquid variable was evaluated by Bülow et al.41 These investigators evaluated the potential benefit of carbonated liquids in the rate of penetration/aspiration, the speed of swallowing (pharyngeal transit time), and post-swallow residue. Carbonated thin liquid resulted in less penetration into the airway than noncarbonated thin liquid, faster pharyngeal transit than thick liquid, and less residue than thick liquid. This finding is intriguing, but clinicians must remember that this is a study, like those described in the preceding section, of the immediate effect of carbonated liquids during the fluoroscopic swallowing examination and does not necessarily translate directly into a proven benefit from use of carbonation as a treatment approach. Taste may be another bolus characteristic with the potential to affect swallowing performance. Logemann et al.42 were among the first to evaluate the impact of taste on swallowing performance in adults with dysphagia. In a comparison of a sour bolus (50% lemon juice and 50% barium liquid) with a regular barium bolus they reported that patients with neurogenic dysphagia demonstrated faster oral onset of the swallow (all patients), decreased pharyngeal delay (stroke patients), and reduced frequency of aspiration (other neurogenic causes). Subsequently, Pelletier and Lawless43 evaluated the impact of citric acid (a sour bolus) and citric acid plus sucrose (a sweet-sour bolus) on the swallowing performance of nursing home residents with dysphagia. They reported that the citric acid solution (2.7%) reduced aspiration and penetration compared with water and that both taste stimuli resulted in increased spontaneous dry swallows following the initial bolus swallow. Additional studies of the impact of taste stimuli on swallowing have focused on healthy volunteers. Chee et al.44 reported that glucose (sweet), citrus (sour), and saline (salty) liquids reduced swallowing speed in healthy adults. Palmer et al.45 compared swallows of sour liquid with water in healthy volunteers and reported that muscle contraction increased (greater electromyographic activity) with the sour bolus but that timing aspects of the swallow did not change across taste conditions. Finally, Pelletier and Dhanaraj46 reported that moderate sucrose (sweet) and high citric acid (sour) and salt concentrations resulted in significantly higher lingual swallowing pressures compared with water. A different outcome is reported in a study from Miyaoka et al.47 These investigators reported no motor changes in swallowing by healthy volunteers resulting from altering taste (sweet, salty, sour, bitter, umami). Thickening liquids may alter the taste of the liquid. Matta et al.48 reported that adding starch-based or gum-based thickeners to common liquids (coffee, milk, apple and orange juice) added either a starchy, grainy, or slick flavor or texture to the liquid and suppressed the base flavor of the beverage. These effects were more pronounced with thicker liquids such as honey-thick consistencies. This observation might help explain the dislike of thick liquids, especially thicker liquids, by adult patients with dysphagia.30 At the least, such observations should encourage clinicians to consider taste and other sensory attributes when recommending thickened liquids for patients with dysphagia. Thickening liquids is a common practice in the management of adult dysphagia. Common reasons for introduction of thicker liquids appear to revolve around clinician perceptions of the patient’s ability to manage liquids orally and to protect the airway from aspiration of thin liquids. Unfortunately, little evidence exists to support this practice. Available evidence does indicate a reduction of aspiration rates in groups of patients when thin liquids are thickened to nectar or honey consistencies during the fluoroscopic swallowing study.20,32 However, evidence also exists suggesting that thickening liquids as a management strategy does not necessarily reduce pneumonia rates.22 Furthermore, evidence also exists that aspiration of thin liquids during the fluoroscopic swallowing study does not necessarily relate to the subsequent development of pneumonia in elderly patients with dysphagia.49 Finally, growing experience with the Frazier Water Protocol35 suggests that patients who aspirate thin liquids may be able to safely drink water with positive health benefit. These clinical and research observations emphasize the need for careful evaluation and continued monitoring of any patient for whom thickened liquids is recommended as a dysphagia management strategy. Clinicians should also consider other liquids modifications such as carbonation and taste variations when contemplating liquid modification as a component of dysphagia management. Similar to liquids, foods may be modified to accommodate perceived limitations in swallowing function in adults with dysphagia. Foods consumed by mouth may be modified for many reasons. Logemann4 describes a study in which patient diet choices were examined. Patients who had been treated for oral cancer were monitored over a 6-month period. Patients tended to eliminate food consistencies that required too much time to eat or consistencies that they were prone to aspirate. These clinical observations suggest that patients will self-modify diet items that are difficult to swallow. Curran and Groher50 described a strategy to modify a hospital’s regular menu to reduce aspiration in patients with dysphagia. Similarly, O’Gara27 and Pardoe28 describe diet modifications intended to promote safe swallowing (minimize aspiration) and adequate nutrition. However, despite the optimism depicted in these early clinical descriptions, more recent clinical research has raised questions about the nutritional adequacy of modified diets. Wright et al.51 reported that older hospital patients eating a texture-modified had lower nutritional intake (energy and protein) than patients consuming a normal diet. These investigators speculated further that other nutrients may also be deficient as a result of the texture-modified diet. Conversely, Germain et al.52 reported nutritional benefit of texture-modified diets over traditional diets in institutionalized elderly patients. Although these two studies focused on different patient groups and evaluated nutritional intake over different periods, the apparent discrepancy between the results suggests that modifying diets for aspiration reduction should not be done in the absence of nutritional consultation. Thus dysphagia clinicians who recommend diet modifications should consult with nutritional specialists to ascertain the nutritional adequacy of the modified diet. Few guidelines exist to aid dysphagia clinicians in making the recommendation for a texture-modified diet or in establishing the optimal level of diet modification. Groher and McKaig53 evaluated swallowing abilities and the type of texture-modified diet in 212 residents in two skilled nursing facilities; 31% of these patients were using a mechanically altered diet. Based on a swallowing examination the investigators recommended changes to oral diets with patient follow-up for 30 days to evaluate response to the new diet level. These investigators reported that 91% of patients examined had been consuming overly restrictive diets. Specifically, these patients could safely ingest diet levels higher (less modified) than they had been consuming on a regular basis; 4% of the patients were on diet levels above what they could safely tolerate, and only 5% were judged to be at the appropriate dietary level. These findings speak directly to two important program management points: (1) A qualified dysphagia clinician should be directly involved in any decision to modify an oral diet, and (2) patients should be monitored and reevaluated at regular intervals to ascertain whether they need diet modification or whether the prescribed level of diet modification remains optimal for the patient’s swallowing abilities. Although this study indicates that modifying a diet level may be more complex than evaluation of airway protection, the results still do not offer clinical guidelines on selecting an appropriate modified diet level. In an attempt to standardize menus and decision processes in the application of modified diets for adults with dysphagia, the National Dysphagia Diet was proposed in 2002.54 The task force developing this diet suggested four standardized levels of diet modification based on assessment of food textures. These four levels are listed in Box 14-2. The task force developing these recommendations performed well in their attempt to recommend a standardized diet modification strategy. In their report they refer to the use of standard assessment tools, provide specific food recommendations for each diet level, describe foods to avoid at each level, describe food preparation approaches, and offer suggestions to enhance patient acceptance of modified diets. The task force also recommended a standard description of thickened liquids to include thin, nectar-like, honey-like, and spoon-thick. However, the efforts of this group did not include specific clinical strategies for use of thickened liquids. Similar to the application of thickened liquids discussed previously, the National Dysphagia Diet represents a solid attempt to provide a standard approach to an important clinical problem, but it also lacks clinical research validation. To date, no significant study has compared the benefits of this standardized approach with other diet modification strategies. However, one study has raised an important question regarding the application of this standardized diet. Strowd et al.55 reported a poor relation between dysphagia foods recommended in the National Dysphagia Diet and the barium materials used to assess patients with dysphagia. Specifically, the viscosity of barium test materials was much greater than the corresponding food recommendations in the National Dysphagia Diet. This observation questions, but does not invalidate, the apparent prescriptive value of National Dysphagia Diet recommendations. Until a high degree of correspondence is developed between the evaluation materials used to make diet recommendations and the food encompassed within those recommendations, clinicians are well advised to follow the advice of Groher and McKaig.53 Patients receiving modified diets should be carefully monitored for acceptance and reevaluated periodically both for safety of the diet (here meaning airway protection) and nutritional adequacy. The core message is that diet modification for adults with dysphagia is not a simple adjustment. The decisions and processes inherent in diet modification demand input, cooperation, and ongoing communication from a team of qualified individuals. One final point merits consideration. Like thickened liquids, texture-modified diets may not be pleasing or acceptable to adults with dysphagia. The National Dysphagia Diet task force acknowledged some of these issues and offered suggestions for improving acceptance. If food looks good, smells good, tastes good, and is presented at the appropriate temperature, it seems logical that patients will be more likely to eat it. The design of “altered foods” for adults with dysphagia is likely to become an important aspect of clinical science and practice. Studies evaluating food characteristics, such as particle size and other physical properties along with specific food content and other factors that may influence food quality, will likely be helpful in developing safe, nutritious, and pleasing diets for adult patients with dysphagia.56 The concept of exercises to stretch, strengthen, or otherwise improve the basic motor properties of muscles in the speech/swallow mechanism is not new.57 Logemann4 indicates that if the patient is aspirating significantly, oral motor exercises may be a better strategy than directly working on swallow function. The rationale for this position is logical. If a patient is continually aspirating, swallow attempts are not maximized (and the patient experiences continued failure). Logemann terms this approach to swallow rehabilitation indirect therapy and offers three foci: (1) exercises to improve oral motor control, (2) stimulation of the swallow reflex, and (3) exercises to increase adduction of tissues at the top of the airway (airway closure). Oral motor exercises include tongue range of motion, tongue resistance, and bolus control activities. Swallow reflex stimulation is advocated via cold thermal-tactile stimulation of the faucial pillars. Airway closure activities incorporate various phonation and “pushing” activities. Some available evidence does support the value of oral motor exercises. Lazarus et al.58 demonstrated that tongue-pushing (resistance) exercises completed with either an Iowa Oral Performance Instrument (IOPI) or tongue blade produced strength increases in young healthy volunteers. Robbins et al.59 reported that a systematic program of lingual resistance exercise improved lingual isometric and swallow pressures (e.g., strength) in a group of 10 healthy older adults. Subsequently, these investigators60 demonstrated that a systematic program of lingual resistance exercise resulted in both increased lingual strength and swallowing ability in a group of 10 poststroke patients with dysphagia. Hagg and Anniko61 demonstrated that a program of resistive lip training improved lip strength and swallow ability in stroke patients with dysphagia. These studies represent evidence that oral motor exercises, specifically lingual and labial resistance exercises, have the potential to strengthen weak swallowing musculature and improve swallow function. To date, little or no evidence has emerged to support other aspects of oral motor exercise. However, as described later in this chapter, exercise principles are being increasingly applied to dysphagia therapy in a variety of approaches. The voluntary breath hold, supraglottic swallow, and super-supraglottic swallow maneuvers are techniques designed to protect the airway from aspiration of food and/or liquid by closing the airway before swallowing. In the case of the two supraglottic swallow techniques, a voluntary cough is executed after the swallow to clear any residue from the vocal folds. The difference between these two maneuvers is the degree of effort in the preswallow breath hold. As implied by the name, the super-supraglottic swallow requires an effortful breath hold, whereas the supraglottic swallow requires a breath hold with no extra effort. The extra effort in the super-supraglottic maneuver is needed to facilitate glottal closure. Glottal closure is one of the earliest aspects of the swallow62; thus techniques that facilitate glottal closure in patients who aspirate may contribute to reduced aspiration. Endoscopic inspection has revealed that healthy adults may not completely close the glottis during a voluntary breath-hold maneuver. Estimates range from 57% to 82% of healthy volunteers who completely close the glottis with a voluntary breath hold.63–65 Adding effort and/or vocalization to the voluntary breath-hold maneuver increases the likelihood that the glottis will be closed.63,65,66 Figure 14-4 demonstrates the difference in glottal closure pattens between a simple breath-hold maneuver and a forced or effortful breath-hold maneuver. Figure 14-4, A demonstrates the glottal closure pattern associated with a simple breath-hold maneuver (e.g., voluntary breath hold or supraglottic swallow). The primary feature is the horizontal (right to left) movement of the arytenoid cartilages and vocal folds to close the airway. When complete, this pattern may be effective in accomplishing airway protection during swallowing attempts. Adding effort to the breath-hold maneuver increases the probability of complete glottal closure. Figure 14-4, B demonstrates the glottal closure pattern associated with a forceful breath-hold maneuver (e.g., super-supraglottic swallow). Note that in addition to the horizontal closure pattern observed in the supraglottic swallow, the arytenoids move anteriorly approximating the petiole of the epiglottis. This movement results in more complete closure of the entire supraglottis rather than closure at the level of the vocal folds only. Of interest is the observation that these two glottal closure patterns (horizontal and anterior) reflect stages in glottal closure in the normal swallow. As demonstrated in Video 14-5, slow-motion analysis of the normal swallow reveals that the glottis is initially closed by the horizontal (medial) movement of the vocal folds. Subsequently, with laryngeal elevation the arytenoid cartilages move forward to approximately the petiole of the epiglottis. Magnetic resonance imaging has demonstrated that complete closure of the larynx is obtained at the point of maximum laryngeal elevation in the normal swallow.67 These closure patterns are reflected respectively in the voluntary breath-hold, supraglottic, and super-supraglottic swallow maneuvers.
Treatment for Adults
WHICH TECHNIQUES AND WHAT TO CONSIDER
MANAGING DYSPHAGIA SYMPTOMS: ADJUSTMENTS, COMPENSATIONS, AND MODIFICATIONS
General Postural Adjustments
Head Postural Adjustments
Head Extension
Head Flexion–Chin Tuck
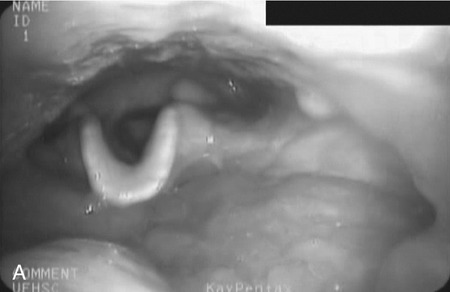
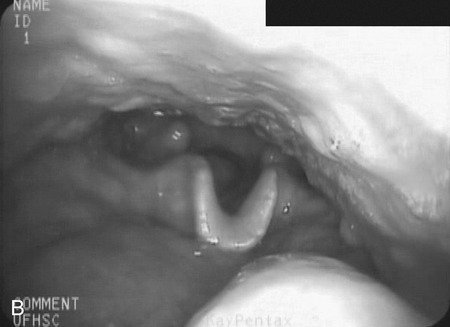
Head Rotation–Head Turn
Thickening Liquids and Modifying Diets
Thickened Liquids: Pros and Cons
ASPIRATION
Amount
Thin
Nectar-Thick
Pudding
5 mL
20%
5%
5%
10 mL
20%
15%
20%
RESIDUE
Amount
Thin
Nectar-Thick
Pudding
5 mL
65%
70%
80%
10 mL
65%
80%
90%
Additional Impact of Thickened Liquids on the Swallow Mechanism
Other Liquid Modifications
Texture-Modified Diets
CHANGING THE SWALLOW: ACTIVE THERAPY TECHNIQUES
Improving the Mechanism: Oral Motor Exercises
PROTECTING THE AIRWAY: BREATH HOLD AND SUPRAGLOTTIC AND SUPER-SUPRAGLOTTIC SWALLOWS
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Abdominal Key
Fastest Abdominal Insight Engine