Totally Extraperitoneal Inguinal Hernia Repair
Kinga A. Powers
Daniel B. Jones
Introduction
Laparoscopic inguinal herniorrhaphy was initially described by Ger in the early 1980s. Since that time, laparoscopic herniorrhaphy has evolved on the basis of traditional open approaches introduced in the 70s by Stoppa, Nyhus, and Wantz utilizing a posterior placement of mesh over the entire inguinofemoral region. Initially laparoscopic intraperitoneal onlay mesh was used; however, exposed intraabdominal mesh raised concern for adhesions. Now, most laparoscopic hernia repairs use the placement of synthetic material into the preperitoneal space. The two laparoscopic inguinal herniorrhaphies performed today are the transabdominal preperitoneal approach (TAPP) and the totally extraperitoneal (TEP) approach. In these repairs, the myopectineal orifice is approached posteriorly and allows for inguinal, femoral, and obturator hernia repairs to be performed simultaneously.
McKernan and Laws were first to report a successful TEP repair in 1993. In the TEP hernia repair the preperitoneal dissection allows for surgical mesh placement over all potential groin hernia defects without entering the abdominal cavity. Although more difficult to master and more costly, there are several advantages of the TEP repair as compared to traditional open techniques of inguinal herniorrhaphy. With TEP, there is less postoperative and long-term neurologic pain and hence shorter convalescence, fewer hematomas, and deep space infections while the recurrence rates remain equivalent to open techniques. When compared to TAPP, TEP offers shorter operative times, especially for bilateral hernias, and decreases the risks of vascular, bowel, and bladder injuries as well as bowel obstructions, adhesions, or fistula formation potentially associated with intraperitoneal dissection and intraperitoneal mesh exposure. It is understandable, therefore, why laparoscopic surgeons often choose TEP as their approach to inguinal herniorrhaphy.
Anatomy of the Inguinal Preperitoneal Space
Clear understanding of the inguinal preperitoneal space anatomy is fundamental in performing the TEP repair. Initial careful anatomical studies of the inguinal region by Bassini, Halsted, Chester, and McVay allow for the current detailed understanding of the groin anatomy, critical in preventing neurovascular and organ injuries.
The preperitoneal space is bounded internally by the peritoneum and externally by the transversalis fascia and the rectus abdominis muscle. Fat, blood vessels, lymphatics, nerves, and the spermatic cord or the round ligament of the uterus all course through this space. The spermatic cord contains the cremasteric muscle fibers, the testicular artery and veins, the genital branch of the genitofemoral nerve, the vas deferens, the cremasteric vessels, the lymphatics, and the processus vaginalis. The vas deferens arises from the seminal vesicle and tracks medial to lateral in the preperitoneal space. The vas deferens courses over Cooper’s ligament, the external iliac vessels, and the iliopubic tract, joining the spermatic cord medially at the deep inguinal ring just inferior and lateral to the inferior epigastric vessels. These three structures form the so-called “Mercedes-Benz” sign.
The peritoneum drapes over the deep aspect of the abdominal wall covering the remnant of the urachus, the obliterated umbilical arteries, and the inferior epigastric vessels to form the median, medial, and lateral umbilical ligaments, respectively. Between and in close proximity to the inferior aspect of lateral umbilical ligaments lies the bladder.
The inferior epigastric vessels branch from the external iliac vessels and lie medial to the internal inguinal ring serving as an important landmark during the TEP repair. From the preperitoneal perspective one recognizes indirect inguinal hernias as lateral to the inferior epigastric vessels, whereas direct hernias occur medial to the inferior epigastrics. When preperitoneal fat herniates through the internal inguinal ring it is known as a cord lipoma and may mimic an indirect hernia. A femoral hernia can also be easily identified in the femoral canal bound laterally by the femoral vessels, medially by the lacunar ligament, anteriorly by the iliopubic tract, and posteriorly by Cooper’s ligament.
Entering the internal ring laterally are the testicular vessels. The testicular vessels and the vas deferens at the internal ring form the apex of a theoretical triangle commonly referred to as the “triangle of doom.” Within this triangle lie the external iliac artery and vein, as well as the genital and femoral branches of the genitofemoral nerve, hidden under peritoneum and transversalis fascia, placing them at high risk of injury. The so-called “triangle of pain” lies lateral to this and its apex is formed inferomedially by the testicular vessels and superolaterally by the iliopubic tract. Within this triangle lie the femoral branch of the genitofemoral nerve, the femoral nerve, and the lateral cutaneous femoral nerve. Stapling of these structures during a laparoscopic hernia repair results in painful neuralgias and should be avoided.
Laparoscopic approaches with TEP and TAPP offer an advantage to open inguinal hernia repairs in bilateral hernias as well as in recurrent hernias status post-open mesh repair. In recurrences from open hernia repairs, scar tissue can be avoided, and dissection in fresh tissue planes from the preperitoneal approach may allow for better inspection of the entire myopectineal orifice for defects. In contrast, recurrences from prior laparoscopic repairs should be repaired through an open approach.
The TEP approach is especially useful in patients who seek an early return to vigorous physical activity. Any increase in intraabdominal pressure post-repair will push mesh into position rather than increase any wound complications as the case may be with open repairs.
Patients with unilateral, bilateral, or recurrent inguinal hernias who can tolerate a general anesthetic are candidates for a TEP repair. On the other hand, patients with comorbidities who are poor candidates for a general anesthetic may be best served by an open inguinal hernia repair under spinal or regional anesthesia. Nonetheless, TEP has been successfully performed under spinal anesthesia when it is possible to sufficiently relax the rectus muscle and allow for preperitoneal CO2 insufflation with low pressures.
Contraindications to a TEP repair include any local or systemic infections that preclude synthetic material use as the risk of mesh infection and need for further surgery to evacuate the infected material is too great under such conditions. Another relative contraindication is a planned or high future risk of a pelvic or extraperitoneal procedure such as radical prostatectomy.
Previous lower abdominal surgery may present a challenge for a laparoscopic surgeon, however is not an absolute contraindications to a TEP repair. If performed carefully,
TEP is still feasible even with a lower midline, a right lower quadrant appendectomy, or Pfannenstiel incisions. Since scaring may render separation of the posterior rectus from the peritoneal surface more difficult, a higher rate of conversion to TAPP or open procedures needs to be anticipated under those circumstances. In addition, a higher rate of visceral injuries must be taken into consideration. In patients with lower abdominal scars, any resistance during initial dissection of the preperitoneal space should alert the surgeon of a potential problem and lead to altering or aborting the TEP procedure.
TEP is still feasible even with a lower midline, a right lower quadrant appendectomy, or Pfannenstiel incisions. Since scaring may render separation of the posterior rectus from the peritoneal surface more difficult, a higher rate of conversion to TAPP or open procedures needs to be anticipated under those circumstances. In addition, a higher rate of visceral injuries must be taken into consideration. In patients with lower abdominal scars, any resistance during initial dissection of the preperitoneal space should alert the surgeon of a potential problem and lead to altering or aborting the TEP procedure.
In cases of strangulated, incarcerated, or large scrotal hernias, an open or a TAPP approach may be a better alternative to TEP. With the blind balloon dissection required for the TEP technique there is a risk of injury to the contents of the incarcerated hernia sac. In such cases, a modified approach of the TEP technique can be used and has been described; however, a more conservative approach is to use an open or TAPP technique if a hernia does not reduce itself spontaneously with a full relaxation of the abdominal wall.
Currently both, TAPP and TEP laparoscopic approaches are acceptable methods of inguinal hernia repair. The conversion from TEP rate has been reported at around 5%. Consequently, surgeons who use the TEP approach need to be equally as proficient with the alternative TAPP and open methods of inguinal hernia repair.
A complete history and physical examination allows for a proper hernia diagnosis and delineation of possible comorbidities and contraindications to a TEP repair. The patient is examined while standing and supine for both inguinal and femoral hernias on both left and right sides. Masses other than hernias in the groin must be ruled out. This can usually be done by physical examination or with the aid of computed tomography or ultrasound imaging. In the case of associated symptoms of fever, tachycardia, exquisite tenderness on groin palpation, erythema of the overlying groin skin, leukocytosis, and/or obstructive symptoms, the incarcerated hernia is likely strangulated and warrants immediate open operative intervention instead of any laparoscopic exploration.
Once a diagnosis is made surgical management of inguinal hernias is discussed with the patient. A clear disclosure of the benefits, pertinent risks of both open and laparoscopic approaches is critical. The possibility of conversion of the TEPP to a TAPP or open repair needs to be explained to the patient. The major intraoperative risks common to both laparoscopic and open inguinal hernia repairs include neurovascular injury (such as lateral femoral cutaneous nerve or common iliac artery injury), injury to other organs such as bladder, bowel, or spermatic cord and its structures. Postoperative complications include urinary retention, groin hematomas, transient or chronic neuralgias, testicular injury, postoperative wound or mesh infections, and hernia recurrence. More specific to the laparoscopic repair as opposed to the open repairs are trocar site complications (hernia or hematoma), and rare risks from CO2 insufflation (hypotension due to elevated intraabdominal pressure, hypercapnea, subcutaneous emphysema, air emboli, pneumothroax, and increased peak airway pressures during surgery). With the TEP approach, the intraperitoneal dissection is avoided and the risks of bowel obstruction secondary to intraabdominal adhesions, mesh adhesions, or visceral injury are minimized as compared to the TAPP procedure. In theory, TEP may avoid the cardiorespiratory alterations associated with creating a pneumoperitoneum, but there have been reports of respiratory acidosis associated with a pneumopreperitoneum.
Operating Room Setup and Patient Preparation
The operating room and equipment are prepared with the appropriate laparoscopic instrumentation and surgical mesh of various sizes available as chosen by the surgeon. In addition to standard open surgical instruments, laparoscopic equipment routinely required for the TEP procedure includes a balloon dissecting device for preperitoneal dissection, a structural balloon trocar or a Hasson type trocar, a 30° laparoscope, two 5 mm trocars and two atraumatic graspers, laparoscopic scissor, a 5 mm clip applier, cautery and a tacking device. Also available, but rarely needed, should be suction irrigator, endoloops, and a Verres needle.
A motorized operating room table is used with capability of placing the patient in Trendelenburg position when required. Two video monitors are positioned at the foot of the patient’s bed and at eye level of the operating surgeon and their assistant. The surgeon stands opposite to the side of the hernia being repaired. One assistant is required and typically holds the camera from the same side of the hernia being repaired. The patient is positioned supine with both arms tucked; alternatively, one arm is tucked on the opposite side to the hernia for a unilateral procedure. This allows the surgeon adequate mobility throughout the case and room to maneuver while placing and fixing the surgical mesh. Generally, the larger or more symptomatic hernia is repaired first before the opposite side is explored.
Although antibiotic prophylaxis has been controversial in both open and laparoscopic hernia mesh repairs, the authors favor prophylactic antibiotics to cover skin flora as to minimize skin and mesh infections (cephalosporin is the most common choice). Preoperatively, the patient empties their bladder; alternatively a Foley catheter is placed under sterile conditions and generally removed at the end of the procedure prior to reversal of anesthesia. The abdomen is prepped and draped from just above the umbilicus to below the pubis. Some surgeons prefer to prep the scrotum as well for the possibility of manipulation during the procedure. Should the spermatic cord be difficult to distinguish, gentle traction on the scrotum may aid in bringing it into view.
The procedure as mentioned is performed under a general anesthetic with full relaxation of the abdominal wall. An appropriate time-out should be performed to identify the patient and the side of the procedure correctly prior to making a skin incision.
A horizontal 10 mm incision is fashioned just below the umbilicus, slightly off the midline towards the side of the hernia being addressed first. The subcutaneous fat is cleared with a combination of cautery and blunt dissection down to the anterior rectus sheath fascia. The anterior fascia is then incised without injuring the belly of the rectus muscle or the small blood vessels often located just anterior to it. Metzenbaum scissors are used to extend the incision in the anterior sheath to approximately 10 mm. The belly of the muscle is bluntly separated from the posterior sheath using a Kelly (Fig. 16.1). Two S retractors are used to gently sweep the muscle laterally and separate any muscle attachments to the posterior sheath thus creating space in the preperitoneal location for the introduction of the dissecting balloon. It is important to remember that in the midline, anterior and posterior rectus sheaths fuse and it is easy to penetrate the posterior rectus into the peritoneal cavity. In the case that the peritoneum is breeched during the initial incision or passage of the dissecting balloon, one may attempt to salvage the TEP procedure by initiating preperitoneal dissection on the opposite side with a bilateral dissection balloon. Alternatively, conversion to a TAPP is an acceptable option preferable to an open conversion.
Prior to the balloon dissection of the preperitoneum the patient is fully paralyzed and positioned in mild Trendelenburg position. The balloon trocar is passed aiming at the anterior part of the symphysis pubis as to avoid injury to the peritoneum or the bladder posteriorly. The dissector is passed along the anterior surface of the posterior rectus sheath and advanced to the pubis, inferiorly past the arcuate line (line of Douglas), where the posterior sheath ceases to exist (Fig. 16.2). Once the pubic bone is reached, the balloon dissector is inflated under direct visualization through the laparoscope to create a working space. Usually 30 to 40 puffs are adequate to insufflate the preperitoneal space bilaterally to the iliac crest. The correct plane of balloon dissection allows for visualization of the peritoneal layer, the pubis, and Cooper’s ligaments as well as inferior epigastric vessels anteriorly. If bowel loops or omental fat come into view a peritoneal tear has occurred.
The dissecting balloon is desufflated, removed and a 10 mm Hasson type trocar is placed at the infraumbilical location, alternatively a structural balloon may be used. The preperitoneal space is then insufflated to low CO2 pressure (10 to 12 mm Hg). Additional trocars are then inserted into the preperitoneal space under direct
visualization. Midline placement of the additional trocars minimizes injury to the peritoneum and inferior epigastric vessels laterally. If inferior epigastric vessels are injured during the dissection, they can be clipped with a 5 mm clip applier and/or divided with cautery or a sealing device. For the midline positioning of the two 5 mm trocars, the lowest trocar is placed at least three fingerbreadths above the pubis. The second 5 mm port is midway between the lowest and the Hasson port.
visualization. Midline placement of the additional trocars minimizes injury to the peritoneum and inferior epigastric vessels laterally. If inferior epigastric vessels are injured during the dissection, they can be clipped with a 5 mm clip applier and/or divided with cautery or a sealing device. For the midline positioning of the two 5 mm trocars, the lowest trocar is placed at least three fingerbreadths above the pubis. The second 5 mm port is midway between the lowest and the Hasson port.
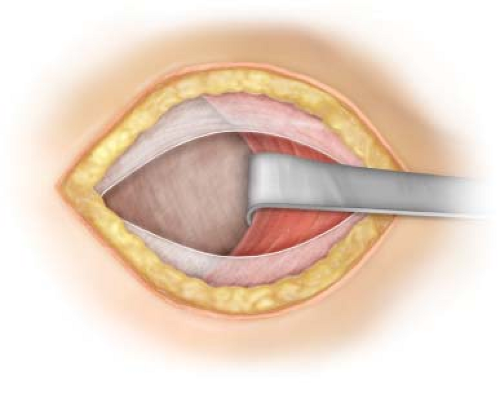 Figure 16.1 Access to the preperitoneal space via infraumbilical anterior rectus sheath incision just lateral to the midline. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


