Musculofascial Flap Reconstruction for Massive Abdominal Wall Hernias
Samuel J. Lin
Charles E. Butler
In the vast majority of cases, there is ample local skin and subcutaneous tissue to close an abdominal wound following various sizes of ventral hernia repair. Rarely, however, there is a need for more complex reconstructive options which are described in this chapter. In these specific cases, a locoregional or free flap reconstruction may be required. A significant evolution of the treatment of large abdominal wall hernias and composite oncologic resection defects has occurred over the last two decades. Although the causes of the abdominal wall defects have largely remained the same (i.e., prior surgery, failure of musculofascial healing, perioperative conditions leading to an open abdominal wound, and tumor extirpation), treatment paradigm has evolved. With the increase in gastric bypass procedures the patient profile of recurrent hernia patients has also evolved over time with more hernia patients who have underwent previous open gastric bypass surgery. The basic principle of abdominal wall reconstruction is to achieve a secure musculofascial repair with stable overlying skin closure. Ideally, the reconstruction of abdominal wall defects occurs in a sterile environment. Unfortunately this is not always the case, particularly in patients with open cutaneous wounds, ostomies, transection of the gastrointestinal tract, mesh infections, or fistulas. The most abdominal defects have sufficient skin for primary advantage and closure, however, some defects require skin to be recruited from remote locations. The use of the fascial component of a tissue flap for musculofascial reconstruction has several disadvantages including the risk of hernia/bulge, paucity of good-quality durable fascia and potential compromised vascularity on the skin portion of the flap with the fascial inset. We believe that reconstitution of the musculofascial and overlying skin defect (when necessary) is optimally performed with two separate techniques.
Full-thickness reconstruction of the abdominal wall is required when the multiply recurrent hernia or ablative abdominal procedure has rendered a significant area of the
musculofascial and cutaneous abdominal wall skin untenable for primary closure. These specific cases occur when there is skin graft directly adherent to the bowel, the abdominal skin/subcutaneous layer is of poor quality secondary to previous incisions or prior radiation, or there exists a large scarred area that has healed secondarily from a prior open abdomen situation. The operative plan should consist of two primary anatomic considerations; those elements that will comprise the musculofascial repair and those components that will comprise the skin reconstruction.
musculofascial and cutaneous abdominal wall skin untenable for primary closure. These specific cases occur when there is skin graft directly adherent to the bowel, the abdominal skin/subcutaneous layer is of poor quality secondary to previous incisions or prior radiation, or there exists a large scarred area that has healed secondarily from a prior open abdomen situation. The operative plan should consist of two primary anatomic considerations; those elements that will comprise the musculofascial repair and those components that will comprise the skin reconstruction.
Most often, the musculofascial repair is achieved by the use of synthetic or biologic mesh depending on the patient and defect characteristics. Prior to the development of new implantable materials and advanced surgical techniques, routine use of uncoated synthetic mesh in primary and recurrent hernia repair was the mainstay of treatment. This technique employed in placement of mesh as an underlay, overlay, or interpositional repair. Alternatively, defects were closed with component separation originally popularized by Ramirez et al. However, the success and longevity of this repair relates to the ability of the patient’s wound-healing capacity to resist infection, the native state of the remaining surrounding musculofascial anatomy, and underlying medical status of the patient. Leber et al. reported that in a case series of 200 patients, the use of a large macroporous mesh incurred a high incidence of recurrent hernias (16%), infection cutaneous extension (16%) or fistula eventual explantation (16%) of the mesh. Few other studies report long-term complications after prosthetic incisional hernia repair. Following removal of infected mesh, patients are frequently left with granulating open abdominal wounds that often underwent temporary closure with resorbable mesh, dressing changes, and subsequent skin graft placement. Delayed permanent reconstruction 6 to 12 months later was challenging owing to continued loss of musculofascial domain, relative skin deficiency, and the need to remove the skin graft without causing an enterotomy.
Treatment of large recurrent abdominal wall hernias with poor-quality overlying skin may be analogous to a large composite wall resection defect. In particular, primary skin close may not be possible in a previously skin-grafted abdominal wall with a large surface area. In these cases, well-vascularized tissue from regional flap tissue is often required for reconstruction.
The skin reconstruction is achieved by transposition of skin and subcutaneous flap tissue from a regional or distant donor site. Thigh-based myocutaneous or fasciocutaneous flaps are excellent options for this purpose owing to a relatively large surface area of skin available and acceptable donor site morbidity. The musculofascial repair is generally performed with surgical mesh and/or without component separation.
For elective, recurrent hernia repair, and planned regional flap reconstruction of the abdominal wall, patients will have had a preoperative medical evaluation and optimized for their nutritional status. Dunne et al. have reviewed independent factors for hernia repair and found chronic obstructive pulmonary disease (COPD) and low preoperative albumin to be independent predictors of wound infection. Additional preoperative considerations include a patient’s preoperative pulmonary function, history of tobacco use, and general medical condition.
When primary closure of the skin and subcutaneous tissue is not an option following musculofascial repair of the abdominal wall, several other options are available. Tissue expansion of the abdominal wall skin is an option when planning for elective hernia repair with skin deficiency. In this technique, tissue expanders are placed lateral to the location of the hernia to expand the skin/subcutaneous layer. These devices are generally placed above the musculofascial layer to directly expand the skin and subcutaneous layer over several weeks to months prior to definitive hernia repair. Tissue expansion is used in many locations around the body; however, there is a risk of wound infection, contamination of tissue expanders, and wound separation leading to the loss of expanded skin, particularly if there is an adjacent open wound or ostomy. In addition, the successful completion of tissue expanded skin often requires several months
prior to hernia repair and preoperative planning time with the requirement of multiple procedures. Lastly, the efficiency of tissue expansion with abdominal tissue expanders is not high since the musculofascia is not rigid and some of the device expansion is transmitted toward the peritoneal cavity rather than expanding the overlying skin.
prior to hernia repair and preoperative planning time with the requirement of multiple procedures. Lastly, the efficiency of tissue expansion with abdominal tissue expanders is not high since the musculofascia is not rigid and some of the device expansion is transmitted toward the peritoneal cavity rather than expanding the overlying skin.
Musculofascial Reconstruction
When component separation is not enough to close the abdominal wall primarily, mesh is usually employed. The choice of synthetic versus bioprosthetic mesh is an important consideration. Macroporous synthetic mesh, such as polypropylene mesh, is frequently used for musculofascial repair in hernia repair. In general, synthetic mesh should only be used in defects without bacterial contamination. Any open abdominal wound, regardless how small, has an increased risk for mesh infections. In the setting of current or recent infection, a synthetic mesh is often considered to be contraindicated. There are several bioprosthetic mesh options available which are decellularized tissue matrices derived from human or animal tissues, most commonly from dermis. Human acellular dermal matrix (HADM) has been used for hernia repair for over a decade but when used in a bridging technique may cause laxity or bulge of the mesh as it remodels. Newer porcine materials have demonstrated good results in animal and human studies. These materials can be differentiated by the presence or absence of chemical cross-linking during their processing. Non–cross-linked porcine acellular dermal materials have been shown to have better biologic regenerative properties than those that undergo chemical cross-linking. Regardless of the operating surgeon’s preference of bioprosthetic mesh, most have some resistance to bacterial infection and limitation of visceral adhesions to the repair site compared to synthetic macroporous mesh. Different materials may have varied cellular incorporation for the surrounding tissues, risk of seroma formation, and tensile strength over time. However, high-level evidence comparative data has not elucidated each of these properties in long-term human studies for the bioprosthetic meshes. Bioprosthetic mesh may tolerate hostile wound environments and conditions. In fact, in a prospective trial repair of infected and contaminated hernias (RICHs) non–cross-linked porcine acellular dermal matrix tolerated bacterial contamination and cutaneous exposure relatively well and none of the 80 patients involved required explantation of the bioprosthetic at 1 year follow-up.
Musculofascial reconstruction followed by flap reconstruction is utilized for settings when there is skin and subcutaneous deficit. Biologic mesh has characteristics of being able to be successfully used in the setting of bacterial contamination and tolerates cutaneous exposure well generally without need for explantation as it becomes revascularized and remodeling by the surrounding tissues. In conjunction with flap reconstruction, human acellular dermal matrix (HADM) is useful for complex abdominal wall fascial reconstruction because of its low visceral adhesion rate, low infection rate, and ability to provide a healed wound through revascularization and cellular infiltration even in the setting of cutaneous exposure. In conjunction with flap reconstruction, using biologic mesh for musculofascial reconstruction prior to flap reconstruction of the skin and soft tissue defect has potential advantages over using the fascia from the flap for the same purpose. It provides a separate, independent musculofascial repair that bears the biaxial load of musculofascial tension thus reducing the surface area and flap inset tension of the skin defect. The limitations of HADM are that it can undergo surface area expansion during the remodeling phases in some patients and result in abdominal wall laxity or bulge, particularly if biologic mesh is used to span the fascial defect (bridged repair) rather than providing complete fascial close over the biologic mesh (reinforced repair). For this reason, non–cross-linked porcine acellular dermal matrices (ncl-PADM) may be more commonly used for abdominal wall reconstructions.
Biologic mesh is useful for musculofascial reconstruction when synthetic mesh is contraindicated or unfavorable (direct unavoidable placement over bowel, bacterial contamination, unreliable overlying skin coverage and high risk for wound-healing complications). Biologic mesh is initially avascular and can bear a substantial load without
concern about devascularization or necrosis. In addition, biologic mesh tolerates cutaneous exposure in the face of wound dehiscence generally without the need to be removed, and the resulting wound can often heal spontaneously by secondary intention and re-epithelialization In addition, biologic mesh has been shown to decrease visceral adhesions to abdominal wall repair sites in animal studies and has a relatively low infection rate; biologic mesh is able to revascularize and become infiltrated with host cells.
concern about devascularization or necrosis. In addition, biologic mesh tolerates cutaneous exposure in the face of wound dehiscence generally without the need to be removed, and the resulting wound can often heal spontaneously by secondary intention and re-epithelialization In addition, biologic mesh has been shown to decrease visceral adhesions to abdominal wall repair sites in animal studies and has a relatively low infection rate; biologic mesh is able to revascularize and become infiltrated with host cells.
Prior reports have outlined the risk of recurrent hernia and bulge in patients with HADM repair. Whenever a bridged fascial repair is performed with HADM (rather than primary fascial closure with HADM inlay reinforcement), there is a risk of laxity and bulge with ongoing remodeling of the implant material. However, neither the minor laxity in the reconstructed musculofascial defect nor the hernia in the described small series were symptomatic, progressed in size, or required surgical correction at the last follow-up. Though there are risks associated with recurrent hernia and bulge with HADM mesh repair, the benefits of HADM mesh includes the ability of HADM to resist visceral adhesions, to be placed into a contaminated surgical wound without high risk for subsequent removal, and to become remodeled into the host tissue with cellular and vascular infiltration are useful properties of bioprosthetic mesh in this setting. Due to the potential for laxity we have been using non–cross-linked porcine acellular dermal matrix for musculofascial reconstruction with limited laxity.
Biologic Mesh Inset
When bridging a fascial defect bioprosthetic mesh is inset with #1 polypropylene sutures using a “dual-circumferential” inlay method as previously described. Briefly, this includes full-thickness musculofascial sutures place 3 to 5 cm from the true musculofascial edge, each placed on hemostats to assure that all sutures are inset correctly before tying them. Generally, in these cases the musculofascial defect is too large to close primarily even with bilateral component separation and a bridged repair is performed. After securing the peripheral suture line the true musculofascial edge is secured to the bioprosthetic mesh with interpreted or running monofilament sutures. The biologic mesh is inset into the musculofascial defect under physiologic tension, which reduces repair site musculofascial laxity and minimizes both the musculofascial and cutaneous defect size. With defects extending to bone, the bioprosthetic mesh is anchored with #1 polypropylene sutures through drill holes in ribs, lumbosacral spine, and/or pelvis.
Flap Options
Flap options differ for cutaneous defects in varied locations of the abdomen. For certain vertical midline defects, patients with a relatively supple skin/subcutaneous layer may be amenable to having a bipedicled skin flap performed bilaterally. This flap is a random flap that is supplied by vascular inflow from the superior and inferior bases of the flap; the central portion of the flap is undermined and advanced to the midline from both sides. The donor sites are usually closed with a skin graft and can be cosmetology disfiguring.
Other local flaps of the abdomen include a bipedicled flap, a V–Y flap (Fig. 30.1), a rhomboid flap, a bilobed flap, and a rotational flap. These flaps are based on a random blood supply and require broadly based flaps for vascularity and a tension-free closure. The objective of cutaneous flap coverage of abdominal defects following hernia repair is to have no tension over a newly repaired abdominal wall. All of these local flaps have limited ability to close large cutaneous defects and have donor site scars.
Regardless of the defect size, repair of large, composite abdominal wall defects using bioprosthetic mesh for musculofascial reconstruction and free or pedicled musculofascial lower extremity flaps for replacement of skin and subcutaneous tissue is an option for various settings including contaminated wounds. The inclusion of the rectus femoris (RF), tensor fasciae latae (TFL), and vastus lateralis (VL) may increase vascularity by providing additional myocutaneous perforating vessels. In addition, pliable vascularized
muscle can be used to obliterate areas of potential dead space in the defect. An extension of the skin paddle to the upper border of the patella allows complete dissection of the lateral circumflex femoral (LCF) vessels to their origin, and a pedicled subtotal musculofascial lower extremity flap has the ability to reach the umbilicus and various maneuvers may get them higher. It has been reported that the flap can reach the costal margin in certain patients.
muscle can be used to obliterate areas of potential dead space in the defect. An extension of the skin paddle to the upper border of the patella allows complete dissection of the lateral circumflex femoral (LCF) vessels to their origin, and a pedicled subtotal musculofascial lower extremity flap has the ability to reach the umbilicus and various maneuvers may get them higher. It has been reported that the flap can reach the costal margin in certain patients.
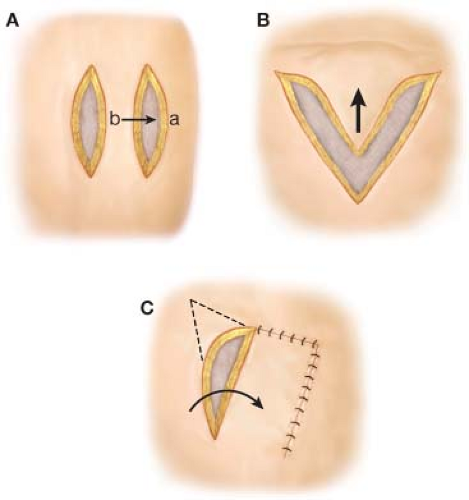 Figure 30.1 Various types of arrangement of local tissue. (A) depicts bipedicled flap reconstruction; (B) depicts a V to Y flap reconstruction; (C) depicts a transposition flap. |
As mentioned, other thigh flaps described for the repair of large abdominal musculofascial defects include fascial extensions of rectus fascia flaps, tensor fascia lata grafts, and anterolateral thigh (ALT) flaps. Nonetheless, the utility of described thigh flaps in abdominal wall reconstruction is limited by a paucity of reliable thigh fascia available. The strongest thigh fascia is the iliotibial band; lateral and medial to this area, the iliotibial fascia becomes thin. Moreover, insetting the fascial component of a musculofascial flap in abdominal wall reconstruction may place tension on the skin paddle, potentially compromising its vascularity. Thus, we strongly believe that musculofascial reconstruction with bioprosthetic mesh prior to inset of a thigh-based flap or other myocutaneous flap is optimal and eliminates these concerns.
Regional Flaps
The latissimus dorsi musculofascial flap is a muscle flap supplied by the thoracodorsal artery and vein pedicle. This broad muscle flap may be elevated from the posterior trunk, with a skin paddle or skin graft over the muscle and used to reconstruct lateral/superior abdominal wall defects. For instance, skin defects occurring in the upper flank are optimal areas for latissimus dorsi flap reconstruction when cutaneous reconstruction is required.
The serratus anterior myofascial flap is a muscle flap option used for smaller defects of the abdominal wall in the lateral and superior portion of the abdomen. The lower three or four muscle slips of the serratus anterior are harvested without risk of significant scapular winging. The vascular pedicle is potentially long (approximately 15 cm) if the subscapular vessels are included with the serratus anterior branch and thoracodorsal blood vessels. The surface area of the serratus anterior flap is usually limited to approximately 10 × 12 cm with respect to utilizing the muscle with a skin graft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


