Fig. 4.1
Intrinsic and extrinsic coagulation pathways
In 1856, Virchow (Fig. 4.2) proposed a triad of events leading to venous thrombosis. He proposed that stasis of blood flow, hypercoagulability of the blood, and damage to the vascular endothelium are associated with thrombosis (Fig. 4.3). It has since become increasingly clear that one or more pathophysiologic factors of Virchow’s triad are a part of any risk leading to the development of DVT.

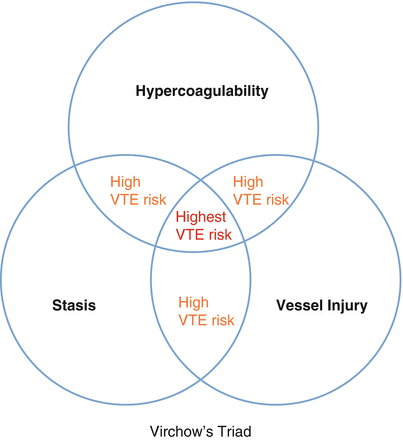

Fig. 4.2
Rudolph Virchow proposed, in 1856, a triad of events (Fig. 4.3) that are necessary to the development of venous thrombosis. With permission from Jatoi I. Surgical Considerations in the Management of Primary Invasive Breast Cancer. In: Jatoi I, Kaufmann M, eds. Management of Breast Diseases, 2010. Springer, New York; pp. 227–241 [99] © Springer

Fig. 4.3
Virchow’s triad
Patients who are hospitalized for acute medical illness and urgent or emergent major surgery are known to be associated with more than a tenfold increased risk for VTE. Most hospitalized patients have at least one risk factor, including immobility , cancer, infection, and/or surgery. When VTE prophylaxis is not used, studies have shown 16 to 55 % of medical and general surgery patients, and 40 to 60 % of patients requiring major orthopedic surgery develop thrombosis. It has been reported that approximately 10 % of hospital deaths are related to PE; many times this disease was not suspected before death [2].
The majority of DVT/PE events are related to specific, identifiable triggering events such as prolonged periods of immobility, hospitalization, major surgery, and trauma. Acquired and/or inherited risk factors are often present in patients who experience a triggering event leading to the development of a DVT or PE.
4.3 Risk Factors for VTE
There are many inherited and acquired risk factors associated with VTE and recurrent VTE (Table 4.1). Strong genetic risk factors that lead to a hypercoagulable state include deficiencies in the anticoagulants antithrombin, protein C, and protein S. Moderate genetic risk factors include factor V Leiden, prothrombin G20210A, and non-type O blood (Table 4.2). Acquired risk factors include age, surgery, obesity, cancer, pregnancy, hormone-based contraceptives, hormone replacement, antiphospholipid syndrome, acute infection, immobilization, indwelling catheter use, paralysis, prolonged travel, smoking, hospitalization, reduced fibrinolysis, and acquired thrombophilia.
Table 4.1
Risk factors associated with VTE
Hypercoagulability | Hypercoagulability | Stasis (abnormal blood flow) | Vessel injury |
|---|---|---|---|
Genetic: | Acquired: | • Immobility | • Surgery |
• Factor V Leiden | • Malignancy | • Polycythemia | • Trauma |
• Prothrombin G20210A | • Chemotherapy | • Atrial fibrillation | • Venipuncture |
• Protein C and S deficiency | • Oral contraceptive use | • LV dysfunction | • Indwelling catheter |
• Antithrombin III deficiency | • Hormonal replacement therapy | • Venous insufficiency | • Atherosclerosis |
• Activated protein C resistance | • Pregnancy | • Varicose veins | • Hypertension |
• Blood group non-O | • Heparin-induced thrombocytopenia | • Venous obstruction due to obesity and/or pregnancy | • Toxins (smoking) |
• Single-nucleotide polymorphism (fibrinogen, factor V, factor XI, other factors) | • Obesity | • Bradycardia | |
• Advanced age (>50 years) | • Hypotension | ||
• Antiphospholipid syndrome | Turbulent blood flow: | ||
• Inflammation | • Heart valve disease or replacement | ||
• Sepsis | • Atherosclerotic plaque | ||
• Nephrotic syndrome | |||
• Inflammatory bowel disease |
Table 4.2
Prevalence of familial and acquired thrombophilia
Condition | Prevalence in Caucasian population, % | Incidence of VTE, % (relative risk) | Incidence of recurrent VTE, % (relative risk) |
|---|---|---|---|
Factor V Leiden | 3–7 | 12–20 (4.3) | 40–50 (1.3) |
Prothrombin 20210A | 1–3 | 3–8 (1.9) | 15–20 (1.4) |
Protein C deficiency | 0.02–0.05 | 2–5 (11.3) | 5–10 (2.5) |
Protein S deficiency | 0.001–1 | 1–3 (32.4) | 5–10 (2.5) |
Antithrombin III deficiency | 0.02–0.04 | 1–2 (17.5) | 2–5 (2.5) |
Plasma markers used to screen for inherited thrombophilia include factor V Leiden mutation, low protein C activity, low protein S activity, and free protein S deficiency. Markers for acquired thrombophilia include D-dimer elevation, fibrinogen elevation, elevation of coagulation factors VIII, IX, and XI, elevation of lupus anticoagulants and homocysteine level, and antithrombin III deficiency (Table 4.2). One study screened bariatric surgery candidates and found that serologic markers occurred more frequently than what would be expected in the general population. Results included D-dimer elevation in 31 %, fibrinogen elevation in 40 %, factor VIII elevation in 50 %, factor IX elevation in 64 %, factor XI elevation in 50 %, and lupus anticoagulant in 13 % [9]. One study identified clinical markers of a hypercoagulable state using rotational thromboelastometry (ROTEM) in patients being prepared for bariatric surgery. ROTEM detects hyperfunctional changes to determine if a hypercoagulable state exists by looking at clot starting time, clot formation time to 20 mm, maximum clot firmness, and clot lysis. Metabolic and inflammatory markers, such as leptin, C-reactive protein, fibrinogen levels, and platelet count, were noted to be significantly higher in the high-risk patients and it was concluded that a hypercoagulable state is associated with central obesity and high fibrinogen levels [10].
4.3.1 Genetic Factors That Increase VTE Risk
Thrombophilia is an inherited blood clotting disorder caused by one or more genetic risk factors or mutations that make a person susceptible to VTE. The risks for VTE are much greater for those individuals with thrombophilia compared to the population at large, particularly for those who also have another risk, such as surgery, hospitalization, or a prolonged bed stay or prolonged travel.
4.3.1.1 Antithrombin III, Protein C, and Protein S Deficiency
Mutations in the genes that produce protein C and its cofactor protein S are found in less than 1 % of the population, while deficiencies in the gene that produces antithrombin are found in roughly 1 in 5000 individuals. There is a tenfold increase in the risk of thrombosis in patients with deficiencies in the protein C, protein S, and antithrombin. The highest risk is seen in patients with antithrombin deficiency.
4.3.1.2 Factor V Leiden
Factor V Leiden is a relatively common mutation in the gene for clotting factor V and is resistant to inactivation by activated protein C, which leads to an increased risk of VTE. This genetic defect is most commonly found among Caucasians of European origin.
4.3.1.3 Prothrombin G20210A
Prothrombin G20210A is single-nucleotide polymorphism in the 3′ untranslated region of the prothrombin gene that leads to increased expression. Roughly 2–3 % of Caucasians have a mutation in the gene that produces prothrombin (clotting factor II). Approximately 6 % of all VTE patients have this mutation, which leads to a threefold increase in the risk of thrombosis.
4.3.1.4 Fibrinogen C10034T
Fibrinogen C10034T is a fibrinogen gamma-chain gene variant associated with increased venous thrombosis.
4.3.1.5 Non-O Blood Type
Certain blood types, especially when combined with certain genetic mutations, constitutes the most significant risk factor for formation of VTE; significantly higher than risk associated with factor V Leiden or prothrombin G20210A alone. Individuals with blood type O have lower von Willebrand factor (vWF) and factor VIII levels than non-O blood group individuals. While hemorrhagic diathesis can be seen in patients deficient in vWF, elevated vWF levels are associated with increased risk of VTE. A two-fold increased risk of a first DVT has been shown in patients with non-O blood type, and VTE recurrence has been associated with blood type B. Non-O blood type also strongly influenced the risk of thrombosis in patients who were factor V Leiden carriers [11].
An individual with a genetic mutation will not necessarily develop a VTE, and fewer than 10 % of those who carry the most common mutations will develop a detectable blood clot each year. At least one-third of patients diagnosed with a DVT will have at least one genetic mutation associated with increased VTE risk [2].
Taking a good family history is vital in a surgeon’s effort to decrease the incidence of VTE in bariatric surgery patients. In almost all cases where there was a presence of an inherited hypercoagulable state, at least one of the parents also had the disorder, and there is a 50 % chance that a sibling or child will have the disorder as well. Other blood relatives, including aunts, uncles, and cousins, may also be affected.
4.3.2 Acquired Hypercoagulable States
Acquired hypercoagulable states make patients more susceptible to VTE and can be seen in patients undergoing surgery or requiring a prolonged hospitalization. More details of how acquired risk factors may affect bariatric surgery patients are reviewed below.
4.3.2.1 Obesity
A systematic review, as well as cohort and case-control studies, demonstrates that obesity doubles the risk compared to that which is seen for healthy weight individuals [12, 13]. One study demonstrated that VTE risk increases with increasing BMI and the associated excess risk is much greater after surgery than without surgery. During a 12-week period without surgery, the incidence rate of VTE per 1000 women with a BMI <25 was 0.10 and ≥25 was 0.19; the corresponding rates in the 12 weeks following day and inpatient surgery were, respectively, about 4 and 40 times higher [14].
The inflammatory state associated with excess body fat and its associated comorbidities creates conditions that increase the risk for VTE. Excess adipose tissue causes hypoxia and increases delivery of inflammatory adipocytokines and free fatty acids (FFAs) to the liver, where coagulation factors are synthesized. FFAs can induce mitochondrial production of reactive oxygen species (ROS), which are cytotoxic and serve as signals to activate endothelial cells and initiate systemic coagulation [15]. Initially, inflammation is confined to the adipocytes , but excess activity overflows into the systemic circulation, where fatty infiltration of the liver, muscles, and vascular endothelium develops. Thus, an inflammatory process now begins in the peripheral tissues, which are not as well equipped to handle the subsequent cytotoxic effects [16]. Loss of body weight has been shown to reduce the concentrations of coagulation factors toward the normal range and improve fibrinolysis [17].
Insulin resistance associated with increasing BMI has been reported to increase the risk of VTE due to the overactivation of the renin-angiotensin system and elevated level of circulating FFAs, which interfere with insulin-mediated glucose uptake. The subsequent hyperglycemia can lead to ROS generation and oxidative stress, which can trigger systemic inflammation and further FFA production [18, 19].
The synergistic effects of obesity with other risk factors increase the VTE risk even further. One study analyzed the risk of obesity associated with oral contraceptives with or without factor V Leiden and found that the incidence of thrombosis was increased 4-fold in individuals taking hormone contraceptives, 7-fold in those with factor V Leiden, and 36-fold in individuals with both risk factors [20].
4.3.2.2 Race and Gender
For reasons that are not completely understood, African-Americans and Caucasians tend to have a greater VTE risk than those whose ethnic background is either Asian or Native American. O blood type is proportionally higher in African-Americans; thus one would expect that African-American individuals would have fewer VTEs. African-Americans have a 30 % higher risk than Caucasians, while Asian and Native Americans have a 70 % lower risk.
Studies demonstrate the risk of recurrent VTE to be higher among men than women [21]. Women have a higher incidence of DVT during their childbearing years, although this risk is still relatively low compared to risk levels for older men and women. However, after the age of 50, men are at greater risk than woman.
4.3.2.3 Age
A number of studies support an association between increasing age and a higher incidence of VTE. The incidence among children (under the age of 14) is quite low, at less than 1 per 100,000 population. Incidence rates increase relatively slowly until the age of 50, and then accelerates dramatically, surpassing 1000 per 100,000 population by the age of 80. The average annual rates of hospitalizations with a discharge diagnosis of DVT, PE, or VTE among adults were 152, 121, and 239 per 100,000 population, respectively. For VTE, the average annual rates were 60 per 100,000 population aged 18–39 years, 143 for persons aged 40–49 years, 200 for persons aged 50–59 years, 391 for persons aged 60–69 years, 727 for persons aged 70–79 years, and 1134 for persons aged ≥80 years [22].
4.3.2.4 Infection and Inflammatory Diseases
Respiratory tract, urinary tract, skin, intra-abdominal infections, and bacteremia diagnosed in hospital or treated in the community were associated with at least a twofold increase in VTE risk. The association was strongest within the first 2 weeks after onset of infection, gradually declining thereafter [23].
Patients with rheumatologic disease have an increased risk for VTE. A meta-analysis evaluating VTE risks in patients with inflammatory arthritis, vasculitis, and connective tissue diseases (including systemic lupus erythematosus (SLE), Sjögren’s syndrome, inflammatory myositis, and systemic sclerosis) demonstrated a threefold higher risk compared to the general population [24].
VTE risk appears to be increased in patients with inflammatory bowel disease (IBD), especially in those patients with trigger events; most often, the trigger event is a hospitalization. A population-based study identified a threefold increased risk for VTE [25]. Patients with IBD are also at an increased risk of recurrent VTE compared to patients without IBD [26]. At the time of a flare, however, this increase in risk was demonstrated to be much more prominent, with the risk being lower during non-hospitalized periods (6.4 per 1000 person-years) than during hospitalized periods (37.5 per 1000 person-years) [27].
4.3.2.5 Nonsteroidal Anti-Inflammatory Drugs
A systematic review and meta-analysis demonstrated a statistically significant increased risk of VTE among nonsteroidal anti-inflammatory drug (NSAID) users. Use of nonselective NSAIDs or cyclooxygenase-2-selective inhibitors (COX2Is) has been associated with an increased risk for VTE. In a population-based case-control study in northern Denmark, use of nonselective NSAIDs or COX2Is was associated with twofold or more increased risk of VTE. Current use was classified as new use (first-ever prescription redemption within 60 days before VTE diagnosis date) or long-term use. Compared to patients who did not use NSAIDs, there was an increased adjusted incidence rate ratio (IRR) associated with current nonselective NSAID and COX2I use with VTE. Recent users had substantially smaller increases than current users [28].
As indicated earlier, rheumatologic patients have a higher VTE risk, which is compounded even further in patients taking NSAIDs chronically for pain management.
4.3.2.6 Smoking
A meta-analysis involving approximately 4 million subjects, and more than 35,000 patients with VTE from 32 observational studies, found a slightly increased risk of VTE for smokers compared to nonsmokers. The risk was higher in studies adjusted for conventional cardiovascular risk factors, especially for BMI. The risk of developing VTE was greater for current smokers than for former smokers, and a dose-response relationship was found for daily smoking and pack-years smoked [29, 30]. A synergistic effect on VTE risk for smoking and oral contraceptive use was demonstrated in one study, reporting an odds ratio of developing VTE for oral contraceptive users of 3.90, which increased to 8.79 when current smoking was added [31].
4.3.2.7 Antiphospholipid Syndrome
The antiphospholipid syndrome is a relatively common acquired cause of venous thrombosis. Antiphospholipid antibodies recognize phospholipid-protein complexes such as β2-glycoprotein I (β2GPI), and prothrombin. These autoantibodies interfere with the physiological mechanisms of the coagulation cascade and fibrinolysis, thus leading to hypercoagulation. It also interferes with the function of platelets, monocytes, and endothelial cells. The protein C pathway is the most important natural anticoagulant pathway, activated in the presence of low concentrations of thrombin. The activated protein C (APC) exerts its anticoagulant effect through proteolytic inactivation of coagulation factors V and VIII. Diagnostic tests performed to confirm antiphospholipid syndrome include lupus anticoagulant, anticardiolipin, and anti-β2-glycoprotein I antibodies (anti-β2GPI) [32].
4.3.2.8 Oral Contraceptives/Hormonal Replacement Therapy
Women using oral contraceptives in their childbearing years and postmenopausal women using hormone therapy are at increased risk for VTE. It is well established that oral contraceptives (OCs) carry a risk of VTE, especially during the first year of use. Studies reveal that some combined OCs containing new-generation and anti-androgenic progestogen (desogestrel, gestodene, drospirenone, or cyproterone) have a higher risk of VTE than older drugs, such as levonorgestrel. Combined oral contraceptives that contain progestogen induce a more pronounced APC resistance than those containing levonorgestrel [33].
Oral contraceptives that contain both estrogen and progestin increase the risk of a blood clot by two- to eightfold. The risk may even be greater with patches that contain transdermal contraceptives, since the amount of estrogen absorbed can be up to 60 % higher.
Postmenopausal women undergoing hormonal therapy also have a higher risk of VTE, with recent large studies suggesting a two- to fourfold increase in risk, with even larger increases in risk for those on high doses of estrogen (greater than 1.25 mg/day) [34].
Women with thrombophilia who also are exposed to oral contraceptives, pregnancy, or hormonal therapy will face a considerably greater risk for VTE.
4.3.2.9 Pregnancy
Although pregnant women are not considered candidates for bariatric surgery, there are rare cases in which a negative pregnancy test was resulted on the day of surgery and then becomes positive within the ensuing postoperative period. Pregnancy increases the risk of DVT fivefold compared to nonpregnancy, with the risk being even greater in the postpartum period [2].
4.3.2.10 Malignancy
About 10 % of patients who present with VTE will have an occult cancer diagnosed within 2 years of the thrombotic episode. The diagnosis of malignancy was established within the first year of presentation of DVT in greater than 75 % of cases and more than 40 % of these cancers were found to be metastatic. Cohort studies and clinical trials suggest that the cancer risk of persons presenting with idiopathic unprovoked VTE is more than three times higher than patients with a provoked VTE, and these patients are typically diagnosed with cancer over the next 5 to 10 years [35].
Cancer patients receiving chemotherapy are at even higher risk. Cancer patients with VTE face much worse outcomes than those with cancer alone. The probability of death within 183 days of initial hospital admission is over 94 % for those with VTE and malignant disease, compared to less than 40 % for those with cancer alone.
The incidence of DVT/PE is substantially higher for cancer patients than for non-cancer patients across all types of major surgery. It is uncertain whether the incidence of VTE decreases to pre-cancer risks or if the risk remains increased in cancer survivors [36].
4.4 VTE Risk and Bariatric Surgery
Patients undergoing elective general surgery, including bariatric surgery, are at relatively low risk of VTE. Specifically, the incidence of VTE is approximately 0.03 % with inguinal hernia repair and laparoscopic cholecystectomy, 0.1 to 0.6 % with operations involving the abdominal wall or appendix, and up 1.7 % with major resections such as esophagectomy, hepatectomy, and splenectomy. Most large series report VTE rates after bariatric surgery of about 0.4 % [6].
Most studies report the highest risk for VTE within the first 3–4 weeks and up to 3 months postoperatively. However, there are some studies suggesting that VTE risk remains elevated for at least 6 months in the postoperative period [37–40].
Using data from nearly 74,000 patients, the Bariatric Outcomes Longitudinal Database (BOLD) demonstrated a VTE incidence of 0.42 % at 90 days, with a risk of approximately 1.5 % after open surgery compared to 0.34 % laparoscopically [41]. The Longitudinal Assessment of Bariatric Surgery (LABS) study reported a 30-day VTE rate of 0.4 % [43].
Analyzing the VTE events in 93 of 27,818 patients (0.33 %), the Michigan Surgery Collaborative (MBSC) reported a DVT rate of 0.21 %, a PE rate of 0.18 %, and both a DVT and PE in 0.06 % [44]. In this dataset there were eight VTE-associated deaths, giving a case fatality rate of 8.6 % and accounting for one-third of all deaths in the registry.
In a large, multi-institutional retrospective chart review of 4293 patients undergoing primary or revisional bariatric surgery over an 8-year period, 57 patients (1.3 %) had a VTE [46]. Pulmonary embolism occurred in 39 (0.9 %), and DVT occurred 18 (0.4 %). Of note, no patients were denied surgery due to risk of VTE. Interestingly, of the patients with PE, 38.5 % had negative duplex studies of the lower extremities. There was only one VTE-related mortality in this study (0.02 %).
Gastric bypass was more likely to result in VTE events than LAGB (OR = 0.31). The incidence of VTE at 6 months for the different operations were as follows: LAGB 0.8 % (n = 616), laparoscopic RYGB 2.7 % (n = 5695), and open RYGB 3.3 % (n = 11,123).
4.4.1 Predictors of VTE Risk After Bariatric Surgery
Univariate and multivariate analysis of the aforementioned study revealed that age, BMI, open, and revisional surgery were predictive of VTE. Comparing different bariatric operations, VTE rates were as follows: 1.1 % of 2945 RYGB patients, 2.9 % of 709 VSG patients, 0.2 % of 467 LAGB patients, and 6.4 % of 171 revisional surgery patients [46].
In the MBSC study, significant risk factors for VTE complication included: previous history of VTE (OR 4.15, CI 2.42–7.08), male gender (OR 2.08, CI 1.36–3.19), operative time more than 3 hours (OR 1.86, CI 1.07–3.24), BMI category (per 10 units) (OR 1.37, CI 1.06–1.75), age category (per 10 years) (OR 1.25, CI 1.03–1.51), and most significant risk factor was procedure type, with duodenal switch carrying the highest risk for VTE [44].
In the BOLD study, the risk of VTE was greater in older patients (HR = 1.04), patients with a higher BMI (HR = 1.05), blacks versus whites (HR = 1.65), pulmonary hypertension (HR = 1.8), lower extremity edema (HR = 2.23), men (HR = 2.32), patients with a history of VTE (HR = 4.96), and prior inferior vena cava filter (HR = 7.66) [41]. The risk of VTE was greater in the patients undergoing gastric bypass than in those undergoing adjustable gastric banding (0.55 % versus 0.16 %). Also, VTE was more frequent when the procedure was performed using an open than a laparoscopic approach (1.54 % versus 0.34 %).
Multiple regression analysis from over 304,000 bariatric surgery patients in the National Inpatient Sample database demonstrated an overall VTE rate of 0.17 %, with a lower VTE rate seen in laparoscopic procedures compared to open procedures (0.13 to 0.45 %) [42]. Alcohol abuse (OR 8.7), open operation (OR 2.5), renal failure (OR 2.3), congestive heart failure (OR 2.0), male gender (OR 1.5), and chronic lung disease (OR 1.4) were associated with a higher rate of VTE.
4.4.1.1 Use of IVC Filters
There is no consensus on the use of IVC filters and what comprises a high enough risk to consider its use. There are studies suggesting that the use of IVC filters in patients undergoing bariatric surgery has been associated with a significantly higher risk for VTE and mortality [44]. However, other studies report safe use of IVC filters with low complication rates [48].
4.4.1.2 Duration of Procedure
There have been several studies looking at procedure time and the risk of VTE. Some of the risk may be associated with technical difficulty of the procedure (i.e., intestinal adhesions, presence of abdominal wall or hiatal hernia), but surgeon skill may play a significant role. One study demonstrated BMI as an independent predictor of operative time, and subsequently an increased incidence of complications and VTE [49]. Another study adjusted for surgeon characteristics and resident involvement, and found that slower surgeons had statistically significant higher rates of complications and VTE [50].
4.4.1.3 Procedure Type
The Michigan collaborative study mentioned in the previous section reported the following VTE rates among different bariatric operations: laparoscopic RYGB 0.65 %, open RYGB 1.04 %, LAGB 0.53 %, VSG 0.74 %, and DS 1.77 %.
In the BOLD study, the 90-day VTE event rates among the various procedures were as follows: LAGB 0.14 % (n = 29,384), RYGB 0.46 % (n = 39,350), sleeve gastrectomy 0.50 % (n = 1806), and BPD with DS 2.16 % (n = 647) [41].
A single-institution database review of 362 biliopancreatic diversion with duodenal switch (BPD-DS) patients found a VTE rate of 3.3 % (n = 12) [47]. Of these 12 patients, 8 presented with DVT, giving a DVT rate of 2.2 %. Four patients presented with PE, giving a PE rate of 1.1 %. All patients in this study received VTE chemoprophylaxis, which was continued for 14 days after discharge in . There were no VTE-related mortalities in this study. Operative time and length of hospital stay were identified as risk factors associated with postoperative VTE complications.
4.4.1.4 Prior History of VTE
4.4.1.5 Impact of Surgical Complications
Complications after bariatric surgery are associated with prolonged hospitalizations and immobilization, and have been shown to play a significant role with increased risk for VTE. A multicenter retrospective analysis looking at patients who underwent bariatric surgery demonstrated a VTE incidence of 0.58 % within 6 months, with a strong association between VTE and surgical complications, and intensive care unit admissions. The majority of complications were anastomotic leaks, abscesses, and infections [38].
4.5 VTE Prevention : Diagnosis and Treatment
Despite significant advances in the prevention and treatment of VTE, pulmonary embolism remains the most common preventable cause of hospital death, responsible for many deaths each year in the USA. Thus, it is vital that efforts continue to be made to find the safest and most effective means of preventing and managing VTE. Practical approaches to the prevention of VTE in surgical bariatric patients are reviewed here.
According to the Agency for Healthcare Research and Quality, the prevention of VTE is the number one strategy to improve patient safety in hospitals [51]. As an example, as part of the Surgical Care Improvement Project, the Center for Medicare and Medicaid Services (CMS) now considers appropriate VTE prophylaxis to be a pay-for-performance quality measure for specific procedures (see Table 4.3) [52–54]. Effective and safe prophylactic measures are now available for most high-risk patients [55–58] and numerous evidence-based guidelines have been published for the prevention of VTE [59–61]. The American College of Chest Physicians clinical practice guidelines recommend VTE prophylaxis by surgical risk groups [60].
Table 4.3
Hospital Quality Alliance/Centers for Medicare & Medicaid Services (CMS) Surgical Care Improvement quality measures for perioperative VTE prevention
General surgery | Any of the following: |
• Low-dose unfractionated heparin (LDUH) | |
• Low-molecular-weight heparin (LMWH) | |
• Factor Xa inhibitor (fondaparinux) | |
• LDUH or LMWH or factor Xa inhibitor (fondaparinux) combined with IPC or GCS | |
Excluded populations: | |
• Patients less than 18 years of age | |
• Patients who have a length of stay >120 days | |
• Burn patients | |
• Patients with procedures performed entirely by laparoscope | |
• Patients enrolled in clinical trials | |
• Patients who are on warfarin prior to admission | |
• Patients whose ICD-9-CM principal procedure occurred prior to the date of admission | |
• Patients whose total surgery time is less than or equal to 60 min | |
• Patients who stayed less than or equal to 3 calendar days postoperatively | |
• Patients with contraindications to both mechanical and pharmacological prophylaxis | |
In the absence of appropriate prophylaxis, the incidence of asymptomatic DVT detected by objective diagnostic screening tests has ranged from 10 to 80 % in various hospitalized medical and surgical groups. From earlier studies, the incidence of fatal pulmonary embolism in the absence of prophylaxis was estimated to be 0.1 to 0.8 % in patients undergoing elective general surgery, which includes bariatric surgery, 2 to 3 % in patients having elective total hip replacement, and 4 to 7 % of patients undergoing surgery for a fractured hip [59].
These estimates are likely lower today because of the increasing use of early ambulation and shorter lengths of hospitalization. However, the incidence of VTE, and in particular fatal pulmonary embolism, remains excessively high, even after hospital discharge.
Most bariatric surgery patients are considered at high risk for VTE given the prevalence of risk factors that promote VTE, including obesity, obstructive sleep apnea/hypoventilation syndrome, and exposure to general anesthesia.
4.5.1 Prevention of VTE
There are two approaches to the prevention of fatal pulmonary embolism:
Primary prophylaxis: Either drugs or physical methods that are effective for preventing DVT.
Secondary prevention : Early detection and treatment of subclinical venous thrombosis by screening postoperative patients with objective tests that are sensitive for the presence of DVT.
However, no single screening method has found universal acceptance for secondary prevention [62, 63]. Accordingly, primary prophylaxis is preferred in most clinical circumstances; it is more cost effective than treatment of complications once they occur [64]. Secondary prevention with screening is reserved for patients in whom primary prophylaxis is either contraindicated or shown to be ineffective.
4.5.1.1 Primary Prophylaxis
Early and frequent ambulation is preferred in surgical patients at very low risk of VTE as a solo measure in the general population.
4.5.1.2 Intermittent Pneumatic Compression
4.5.1.3 Graduated Compression Stockings
Graduated compression stockings (GCS) alone can help prevent DVT, but when combined with other prophylactic methods appear to improve rates of DVT prevention.
4.5.1.4 Inferior Vena Cava Filter
The only widely accepted and validated indications for vena cava filter placement in patients with thromboembolism are an absolute contraindication to therapeutic anticoagulation, and failure of anticoagulation when there is acute proximal venous thrombosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







