The role of lymph node dissection (LND) in the staging and treatment of renal cell carcinoma has long been a topic of debate. The controversy has focused on whether LND is purely an adjunctive staging procedure or has a therapeutic role in the management of this disease. Potential benefits include enhanced staging, better selection for adjuvant therapies/clinical trials, a decrease in recurrence rates, and improved disease-specific and overall survival. This article reviews the available literature on LND in renal cell carcinoma and discusses the potential benefits of aggressive surgical resection in select high-risk patients.
The role of lymph node dissection (LND) in the staging and treatment of renal cell carcinoma (RCC) has been a topic of debate since Robson and colleagues first reported a survival advantage in patients undergoing concomitant radical nephrectomy and complete retroperitoneal LND (RPLND) from the crus of the diaphragm to the bifurcation of the great vessels. The debate has focused on whether LND is purely an adjunctive staging procedure or has a therapeutic role in the management of this disease. The potential benefits of LND include enhanced staging, better selection for adjuvant therapies/clinical trials, a decrease in recurrence rates, and improved disease specific and overall survival.
Approximately 30% to 40% of patients with RCC will either present with distant metastatic disease or develop distant disease after nephrectomy. The presence of lymph node metastasis in patients with locally advanced RCC is one of the strongest prognostic factors influencing survival. The 5-year survival rate of patients with lymph node–only metastasis ranges from 5% to 38%. Depending on the extent of local disease and presence or absence of distant metastatic disease, lymphadenopathy (short-axis size ≥1 cm) detected in the retroperitoneum on cross-sectional imaging harbors metastatic disease in 30% to 70% of patients. In the absence of distant metastatic disease, the incidence of isolated regional lymphatic metastases (pN+M0) in patients staged as clinically node-negative (cN0) with modern cross-sectional imaging is rare and estimated to occur in only 1% to 5% of patients. However, these pathologic rates (pN+) vary considerably based on multiple clinical and pathologic features, whether the patient actually undergoes an LND, and the extent of LND performed. In the absence of distant metastatic disease, nodal metastasis can be detected at higher rates in patients with high stage and grade primary tumors and those with clinical lymphadenopathy.
Because of the infrequent occurrence of isolated lymph node metastasis along with the significant stage migration that has occurred in RCC, using an “all or none” practice will either expose a majority of patients to an unnecessary and potentially morbid procedure or may preclude a portion of patients from undergoing a potentially therapeutic procedure. Although level one evidence does show LND is unnecessary in patients with low-risk primary tumors with clinically negative regional lymph nodes, multiple investigators have attempted to quantify an individual’s risk for nodal metastases based on adverse features. The authors believe that a risk/benefit approach should be applied when deciding on the applicability of a LND in a given patient. This article reviews the available literature on LND and discusses specific subsets of patients for whom aggressive surgical resection involving a lymphadenectomy may be beneficial.
Prospective evidence
The European Organisation for Research and Treatment of Cancer (EORTC) trial 30881 is the only large prospective randomized trial assessing the role of LND in RCC. The investigators randomized 772 patients with cT any N0M0 RCC to radical nephrectomy with or without LND. Patients were excluded if clinically node-positive disease was detected on preoperative CT. The right-sided template included the paracaval, retrocaval, precaval, and interaortocaval nodes, whereas the left side dissection included the preaortic, paraaortic, and left diaphragmatic nodes. With respect to morbidity, recurrence, and survival end points, no differences were found with the addition of LND to a standard radical nephrectomy. Several important characteristics of the study population must be highlighted. This trial did not use a risk-based protocol to guide performance of an LND, and most patients enrolled had low-risk primary tumors (approximately 70% pT2 and 70%–80% grade 1–2). The median tumor size in the LND group was 5.5 cm. Not surprisingly, only 4% of patients were found to harbor clinically occult lymph node disease (only 1% if cN0 by imaging and nonpalpable at surgery).
The EORTC 30881 study provides level one evidence that LND does not have a role in patients presenting with low-risk primary tumors. Because of the stage migration that has occurred in RCC, these results will apply to most contemporary patients. However, it may not be prudent to liberally apply these findings to all patients, and particularly those with high-risk primary tumors, clinically node-positive disease, or those being considered for adjuvant clinical trials.
Staging
If the potential therapeutic role of a properly performed LND is discounted, its use still provides more accurate staging and consequently a more accurate assessment of prognosis. Although multiple trials of contemporary adjuvant therapies are underway, omitting the staging LND in high-risk locally advanced disease may impede the selection of patients for adjuvant therapy and represents a confounding factor when assessing the results of these trials.
In the current American Joint Committee on Cancer staging system (AJCC version 7), lymph nodes are categorized as either pNx, pN0, or pN1. Any positive lymph node, regardless of the number positive, is assigned the pN1 designation, whereas the former pN2 (≥2 + nodes) designation has been removed. The change in version 7 occurred as a result of several series showing a lack of prognostic significance between the pN1 and pN2 designations. In contrast, a recent analysis of patients with isolated retroperitoneal lymph node metastases showed a significant difference in overall (median time not reached vs 21.8 months) and recurrence-free survival (54.7 vs 4.4 months) between the former N1 and N2 designations. Additional prognostic factors of lymph node disease have been implicated, including extranodal extension, size of lymph node, lymph node density, and total number of nodes removed, but are not incorporated into the current staging system.
The number of lymph nodes necessary or the minimal template required to be categorized as pN0 is not defined by the AJCC. As expected, the total number of lymph nodes removed (and examined) correlates with the identification of node-positive disease. Terrone and colleagues examined this relationship in 725 patients undergoing radical nephrectomy for RCC; 84% of the patients also received a variable template lymphadenectomy, with a median number of 9 nodes removed. The rate of pN+ disease was significantly increased when the pathologist examined 13 or more nodes (20.8% N+ vs 10.2% N+ for ≤12 nodes; P = .001). The only independent factor related to the number of nodes removed was the surgeon, with the surgeon performing the highest median number of nephrectomies also having the highest lymph node count. The authors advocated that a minimum of 13 nodes should be excised to adequately stage a patient. Although node count has been advocated as a surrogate marker to define the extent of dissection, multiple technical factors related to pathologic processing can lead to significant variability in total node counts independent of the template of dissection.
Ward and colleagues at the Mayo clinic evaluated whether including pNx with patients staged as pN0 was appropriate when creating prediction models for outcomes after radical nephrectomy in clear cell RCC. On univariate analysis, patients staged as pN0 were more likely to die of RCC than those staged as pNx. However, when accounting for tumor stage and grade in a multivariate model, no difference was seen in cancer-specific survival between patients staged as pNx and those as pN0. The practice at this center has been to use the presence of multiple adverse features in the primary tumor to determine individual risk and whether to perform an LND. Therefore, patients undergoing LND were probably more likely to have adverse features, which prompted the surgeon to stage the retroperitoneum. With the use of this clearly defined approach, the grouping of patients with pNx and those with pN0 seems appropriate. However, this may not be valid in an understaged population when LND is underused. These findings have been further replicated and highlight the role of the primary tumor in defining subsequent disease biology.
Staging
If the potential therapeutic role of a properly performed LND is discounted, its use still provides more accurate staging and consequently a more accurate assessment of prognosis. Although multiple trials of contemporary adjuvant therapies are underway, omitting the staging LND in high-risk locally advanced disease may impede the selection of patients for adjuvant therapy and represents a confounding factor when assessing the results of these trials.
In the current American Joint Committee on Cancer staging system (AJCC version 7), lymph nodes are categorized as either pNx, pN0, or pN1. Any positive lymph node, regardless of the number positive, is assigned the pN1 designation, whereas the former pN2 (≥2 + nodes) designation has been removed. The change in version 7 occurred as a result of several series showing a lack of prognostic significance between the pN1 and pN2 designations. In contrast, a recent analysis of patients with isolated retroperitoneal lymph node metastases showed a significant difference in overall (median time not reached vs 21.8 months) and recurrence-free survival (54.7 vs 4.4 months) between the former N1 and N2 designations. Additional prognostic factors of lymph node disease have been implicated, including extranodal extension, size of lymph node, lymph node density, and total number of nodes removed, but are not incorporated into the current staging system.
The number of lymph nodes necessary or the minimal template required to be categorized as pN0 is not defined by the AJCC. As expected, the total number of lymph nodes removed (and examined) correlates with the identification of node-positive disease. Terrone and colleagues examined this relationship in 725 patients undergoing radical nephrectomy for RCC; 84% of the patients also received a variable template lymphadenectomy, with a median number of 9 nodes removed. The rate of pN+ disease was significantly increased when the pathologist examined 13 or more nodes (20.8% N+ vs 10.2% N+ for ≤12 nodes; P = .001). The only independent factor related to the number of nodes removed was the surgeon, with the surgeon performing the highest median number of nephrectomies also having the highest lymph node count. The authors advocated that a minimum of 13 nodes should be excised to adequately stage a patient. Although node count has been advocated as a surrogate marker to define the extent of dissection, multiple technical factors related to pathologic processing can lead to significant variability in total node counts independent of the template of dissection.
Ward and colleagues at the Mayo clinic evaluated whether including pNx with patients staged as pN0 was appropriate when creating prediction models for outcomes after radical nephrectomy in clear cell RCC. On univariate analysis, patients staged as pN0 were more likely to die of RCC than those staged as pNx. However, when accounting for tumor stage and grade in a multivariate model, no difference was seen in cancer-specific survival between patients staged as pNx and those as pN0. The practice at this center has been to use the presence of multiple adverse features in the primary tumor to determine individual risk and whether to perform an LND. Therefore, patients undergoing LND were probably more likely to have adverse features, which prompted the surgeon to stage the retroperitoneum. With the use of this clearly defined approach, the grouping of patients with pNx and those with pN0 seems appropriate. However, this may not be valid in an understaged population when LND is underused. These findings have been further replicated and highlight the role of the primary tumor in defining subsequent disease biology.
Anatomy and templates
The lymphatic drainage of the kidneys was first described in a cadaveric series by Parker in 1935. These pathways can be highly variable, with occasional primary drainage sites located in the pelvis or intrathoracic regions. Most importantly, the renal hilar lymph nodes are not the initial landing sites in most patients with regional lymphatic metastasis, and therefore isolated sampling of the hilar lymph nodes is inadequate when an LND is being performed. As a general rule, both kidneys will drain to interaortocaval lymph nodes. In addition, the right kidney has primary drainage to the precaval, retrocaval, and paracaval regions, whereas the left kidney drains to the preaortic, retroaortic, and paraaortic regions.
Crispen and colleagues examined the location of positive lymph nodes in a series of 169 patients with high-risk clear cell RCC. Patients were defined as high-risk if they had two adverse primary tumor features. This high-risk protocol uses intraoperative pathologic assessment of the primary tumor for nuclear grade 3 or 4, presence of sarcomatoid dedifferentiation, size of 10 cm or greater, stage T3 or higher, and the presence of histologic tumor necrosis. Of these high-risk patients, 64 (38%) were found to have pathologic positive nodes (pN+) within the retroperitoneum. Disease was never present in a contralateral landing zone without first involving one of the ipsilateral sites or the interaortocaval lymph nodes ( Fig. 1 ). Again, simply performing a hilar LND would have missed most of a significant proportion of pN+ disease.
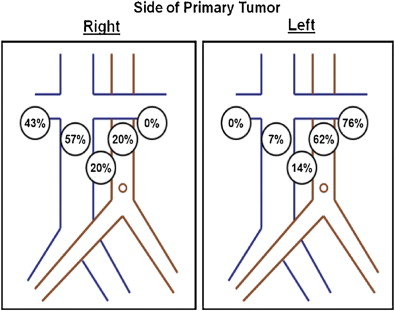
Based on these data, the authors proposed a standardized template for lymph node dissection in RCC encompassing the nodes from the ipsilateral great vessel and interaortocaval regions down to the bifurcation of the great vessels while using intraoperative frozen section of the lymph nodes to guide the expansion of the template to a full bilateral RPLND. An important caveat to this study was that 66% of these patients had clinically enlarged lymph nodes in addition to high-risk primary tumors. Although intraoperative frozen section does seem to be efficacious in providing reliable pathologic diagnoses in patients with clinically positive lymph nodes (≥1 cm), the negative predictive value when assessing for occult lymphatic disease in nonpalpable nonenlarged lymph nodes is unknown.
In an older study by Herlinger and colleagues, the authors examined the outcomes of 511 patients with localized and regionally advanced RCC to determine whether an extended rather than a facultative LND provided any therapeutic benefit. A total of 320 patients received a systematic extended dissection (≥17 lymph nodes removed per patient), whereas 191 had only a facultative dissection (0 nodes in 50%; 1–5 nodes in 30%; >5 nodes in 10%). The incidence of pathologic positive nodes in the extended and facultative groups was 17.5% and 10%, respectively. The 5- and 10-year overall survival rates were significantly different, with a large benefit shown in the extended LND group (5-year, 66% vs 58%; 10-year, 56.1% vs 40.9%). The authors reported that the survival advantage was most pronounced in patients with lower-stage disease (Robson stage 1 and 2). In addition, morbidity was not increased in patients undergoing extended versus facultative LND.
Bex and colleagues recently reported on the feasibility of sentinel node detection in RCC. The investigators used preoperative single-photon emission CT (SPECT) to identify the sentinel lymph node (SN) followed by intraoperative identification with a gamma probe and camera. Although this was a feasibility study of only 20 patients, the SN was identified in 70% of cases. Excision of the SN was performed through either an open (80%) or laparoscopic (20%) approach. Whether this approach can be replicated and would be feasible in patients with regionally advanced RCC to detect clinically occult lymphatic disease will require further investigation.
Morbidity/technique
Although the EORTC 30881 trial did not show any significant differences in operative or postoperative complications, we believe that the addition of an LND to a standard radical nephrectomy conveys a higher risk of lymphadenectomy-specific complications over nephrectomy alone. Multiple factors may contribute to the development of a complication, including the extent of dissection, the presence of bulky clinically positive lymph nodes, medical comorbidities, and individual surgeon experience. Complications associated with LND include injury to major vessels or adjacent organs, bleeding, lymphocele, chylous ascites, and postoperative small bowel obstruction. A standardized system for assessing complications has not been used in comparable cohorts to provide a valid estimation of risk for these procedural-specific complications.
Minimally invasive techniques for treating RCC have increased tremendously over the past 15 years. When compared with its open counterpart, minimally invasive radical nephrectomy may be associated with a decrease in morbidity, particularly postoperative pain and convalescence. As shown by Chapman and colleagues, laparoscopic RPLND is technically feasible in patients with clinically negative regional nodes. Although the template of LND was variable and gradually increased in scope with increasing experience, no differences in complications were noted in patients undergoing laparoscopic nephrectomy versus laparoscopic nephrectomy with LND. Although this seems feasible, considerable experience with minimally invasive techniques is necessary to laparoscopically replicate the LND templates, as recently described by Crispen and colleagues.
Surgical approach does seem to influence the use of LND in patients undergoing radical nephrectomy for RCC. In an analysis of the U.S. Kidney Cancer Study, Filson and colleagues evaluated 730 patients with RCC undergoing open (n = 427, 58%) or laparoscopic (n = 303, 42%) radical nephrectomy for RCC. Lymphadenectomy was performed in only 11% of cases and seemed to be a limited dissection in most of these cases. LND was performed more frequently in patients undergoing open radical nephrectomy versus laparoscopic radical nephrectomy (14.1% vs 5.9%; P <.01) and those with higher-stage, higher-grade, and larger-sized tumors. The potential staging or therapeutic benefit of LND in highly selected cases may be further obfuscated if clinical decisions are based on technique alone. Like in the not-so-uncommon case of patients undergoing laparoscopic nephrectomy in lieu of open partial nephrectomy for T1a masses, surgeons must be wary of supplanting potential oncologic benefits (staging or therapeutic) with the desire to use minimally invasive techniques.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








