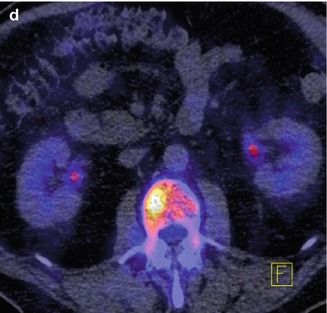
Fig. 48.1
F-18 NaF PET bone scan images of a 87 year old male treated with radical prostatectomy in 1997 for Gleason score 5 adenocarcinoma. He experienced biochemical recurrence, and continued to have rising PSA despite salvage radiation therapy in 1998. There is no evidence of recurrence or metastasis on standard imaging (CT abdomen and pelvis, panel a and MDP Tc 99 m bone scan, panel b). However, on the F-18 NaF PET bone scan, panels c and d, a right-sided L2 metastatic lesion is evident. Other non-neoplastic findings include T11 osteophytic activity, Paget’s disease in the right hemipelvis, and degenerative changes in the knees, all manifested as uptake on the Tc99m bone scan (All images courtesy of H. Jadvar, Department of Radiology, Keck School of Medicine of USC; Support by NIH/NCI Grant R01-CA111613)
With more sensitive imaging and increasing treatment options, men will then potentially spend even longer in the metastatic state. This highlights the important dual goals of managing quality of life in addition to maximizing life span.
Once metastases are identified, whether by newer imaging modalities or traditional ones, men are treated with androgen deprivation therapy (ADT). Given the importance of quality of life, the traditional approach has been similar to that in hormone-receptor positive metastatic breast cancer, relying on hormone therapy initially, often several manipulations, followed by chemotherapy only at progression, in a sequential approach. This chapter will review the biology behind ADT and explore the evolution of the treatment to its current standards, discussing combined blockade versus monotherapy, intermittent versus continuous ADT, peripheral blockade, up-front chemohormonal therapy, and future directions such as combinations of ADT with biologic agents. Supportive care for men undergoing ADT is an important focus, given the increasingly recognized metabolic complications in addition to the many side effects, which impact quality of life. With its dominant proclivity for bony spread, interfering with the signaling between prostate cancer and bone marrow stroma is a high priority, and can have substantial impact by reducing fractures, bone pain, and spinal cord compression.
Androgen Deprivation Therapy (ADT): The Biology Behind the Cornerstone Treatment
Huggins and Hodges were awarded the Nobel prize in the 1940’s for describing the clinical and pathologic responses in men with metastatic prostate cancer who were treated with surgical castration or estradiol [6]. Surgical castration remained a mainstay of treatment until further Nobel award-winning work by Andrew Schally discovered the molecular structure of luteinizing hormone-releasing hormone (LHRH) and the importance of the hypothalamic-pituitary-gonadal axis [7]. This facilitated the subsequent development of synthetic LHRH agonists such as leuprolide and goserelin. Medical castration induced by LHRH agonists was then compared clinically against surgical castration or estrogen, and found to yield equivalent survival [8]. One downside of the agonist approach is an initial flare of testosterone induced by injection of the LHRH agonist, before the down-regulation of LHRH receptors occurs, which ultimately shuts down testicular production of testosterone. This flare can be clinically significant, inducing urinary retention in men with obstructive urinary symptoms or pain in men with bone metastases. A brief period of anti-androgen therapy with flutamide or bicalutamide before the first LHRH agonist injection and during the first few weeks after the injection protects against clinical progression during testosterone flare [9]. Alternatively, new agents, which are pure LHRH antagonists have been developed in order to avoid the flare; these include cetorolix and degarelix. Degarelix is reported to achieve superior testosterone suppression, compared to leuprolide [10], and is not associated with testosterone flare. The clinical significance in terms of cancer control, however, has not yet been established such that both agonists and antagonists are accepted as standard of care. Another pharmacologic alternative to avoid the flare is ketoconazole, an inhibitor of steroid hormone synthesis which blocks both testicular and adrenal production of androgens, and leads to a rapid decline in circulating testosterone levels [11]. The hypothalamic-pituitary-gonadal axis and role of antiandrogens is depicted in Fig. 48.2.
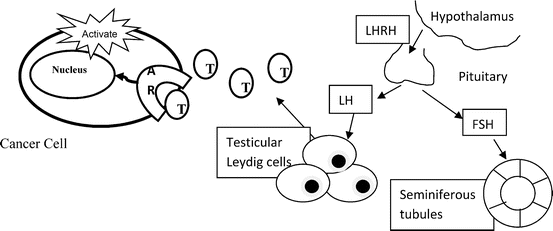

Fig. 48.2
Pituitary-Hypothalamic-Gonadal Axis, and the interaction of testosterone with the androgen receptor at the level of the cancer cell. Luteinizing hormone releasing hormone (LHRH) analogues act as either agonists, leading to cessation of testosterone production by testicular Leydig cells after an initial surge which downregulates LHRH receptors on the pituitary gland with subsequent absence of LHRH stimulation, or antagonists with immediate lack of LHRH stimulation, leading to cessation of testosterone production. Anti-androgens work at the level of the interaction between testosterone (T) and the androgen receptor (AR)
Responses to LHRH analogues occur in the vast majority of men; the majority of patients will have a PSA decline by 28 days into treatment [10] and 80 % of symptomatic patients will experience clinical benefit [12]. However, rising PSA is inevitable, heralding the emergence of castration resistance and clinical disease progression. Multiple mechanisms of resistance have been described, including amplification of the androgen receptor (AR), mutation of the AR and splice variants, changes in AR coactivators, cross-talk or bypass via other growth factor signal pathways including epidermal growth factor receptor and insulin like growth factor, and extragonadal (even intratumoral) testosterone synthesis, reviewed by Attar and colleagues [13]. Interestingly, however, the striking differences among men in terms of time to castration-resistance have not yet been explained. The ability to identify men who will have a sub-optimal duration of disease control with LHRH analogues would facilitate more rapid testing of novel approaches to first-line therapy in metastatic prostate cancer.
Evolution of the Standard Approach to Androgen Deprivation for Advanced Prostate Cancer
Three major classes of drugs make up the androgen deprivation armamentarium: LHRH analogues, which turn off testicular androgen production, androgen receptor antagonists, and androgen biosynthesis inhibitors, which inhibit adrenal and intratumoral conversion of cholesterol into androgens. The last class has typically been utilized as salvage therapy, while the first two classes have been tested as monotherapy and in combination for first-line treatment. Although randomized clinical trial data regarding the following questions are available, the landscape is complicated by additional factors such as quality of life, cost-effectiveness, and metabolic consequences of ADT such that currently there is not one agreed-upon best practice, and both intermittent and continuous therapy, combined blockade and monotherapy are utilized.
Combined Blockade Versus Monotherapy
The rationale for combined androgen blockade began with the recognition that suppressing testicular production of androgens did not lead to the complete absence of androgens, and that all tumors ultimately gained the ability to grow in castrate conditions. In animal models, the addition of androgen receptor blockade to castration therapy enhanced antitumor efficacy. Early clinical trials seemed favorable, and two SWOG trials, 8494 and 8894, were completed to test combined blockade against medical or surgical castration monotherapy [14, 15]. The addition of flutamide to medical castration with leuprolide significantly prolonged survival, 35.6 months versus 28.3 months, two-sided p = 0.035 [14]. However the addition of an anti-androgen to surgical castration did not significantly prolong survival (HR 0.91, 95 % confidence interval 0.81–1.01) [15]. A European (EORTC) trial mirroring the leuprolide trial also documented a survival advantage to combined blockade [16]. Several meta-analyses of combined blockade trials have been published; despite methodological differences, there appears to be consensus that combined blockade is associated with a small but detectable improvement in 5-year overall survival, but questions remain about cost-benefit and quality of life trade-offs [17–19].
Intermittent Versus Continuous
Continuous testosterone deprivation was initially the standard, given that bilateral orchiectomy was the original means of reducing circulating testosterone, leading to permanent absence of testicular androgen production. After the development of LHRH analogues, the ability to reversibly suppress testosterone production fuelled interest in applying therapy intermittently. Using the Shionogi mouse model, Bruchovsky and colleagues [20] demonstrated that the number of castration-resistant prostate cancer stem cells was reduced by applying intermittent therapy, suggesting that cancer cells adapt to the hormone milieu, and suggesting that castration resistance could be delayed by intermittently re-introducing testosterone. Experiments in which prostate tumors were reduced via castration, then transplanted into non-castrate hosts, then re-transplanted into castrate hosts resulted in a fold-fold prolongation of time to castration resistance compared to tumors which remained in the castrate host [21]. In addition to the physiological rationale, there is a strong incentive to utilize intermittent therapy in order to improve quality of life and reduce adverse effects via testosterone recovery during the “off-treatment” period.
Multiple phase II and small randomized trials of intermittent androgen deprivation have documented the safety of the approach, reviewed by Boccon-Gibod and colleagues [22]. These studies document the intermittent experience across a wide range of induction durations, from as little as 3 months to as long as 48 months, and include studies using diethylstilbestrol, LHRH analogue monotherapy, and combined blockade with an antiandrogen. The triggers for resumption of ADT have been similarly variable, although essentially all have been PSA-based, ranging from any rise in PSA to a 50 % increase in nadir, an absolute PSA level >3 ng/mL to >20 ng/mL, and of course clinical progression being an absolute indication to reinitiate therapy regardless of PSA level. Importantly, nearly all patients respond to re-treatment and testosterone levels typically do recover, bringing with them restoration of sexual function for a majority of men. One small randomized trial suggested progression at 3 years was markedly lower for intermittent therapy [23], however larger studies have not borne this out.
Two large, randomized phase III trials comparing intermittent to continuous ADT have issued at least preliminary reports. The South European Uroncological Group (SEUG) treated 766 men with locally advanced or metastatic prostate cancer with cyproterone acetate plus monthly LHRH analogue for an induction period of 3 months [24]. The 626 men whose PSA declined to <4 ng/mL or to 80 % below the baseline value were randomized to intermittent or continuous therapy; those assigned to intermittent therapy stopped treatment at randomization. The re-treatment trigger was two-tiered; PSA >10 ng/mL for symptomatic patients and 20 ng/mL for asymptomatic patients and for patients whose PSA declined to below 80 % of the initial value, a PSA rise >20 % above the nadir triggered the next cycle of ADT. Survival was similar, with disease progression documented in 127 men from the intermittent arm and 107 men from the continuous arm; while there were more cancer-related deaths in the intermittent arm, there were more cardiovascular deaths in the continuous arm. Quality of life was not significantly different in the two arms, except that men on the intermittent arm had greater sexual function. A second multinational trial of intermittent therapy, JPR7, reported preliminary findings at the 2011 annual meeting of the American Society of Clinical Oncology [25]. One thousand three hundred and eighty-seven men with biochemical recurrence were treated with 8 months of LHRH analogue monotherapy. Those who achieved a PSA <4 ng/mL and did not show signs of castration resistance were randomized to intermittent or continuous therapy. Men on the intermittent arm resumed therapy when PSA reached 10 ng/mL and each treatment cycle was 8 months. For the primary endpoint of overall survival, there was no significant difference, and the p value was 0.009 for non-inferiority; as in the SEUG trial there were more prostate cancer deaths on the intermittent arm but more non-prostate cancer deaths on the continuous arm. Men on the intermittent arm spent only 27 % of their time on treatment, which would be expected to yield cost savings; quality of life data are not yet available. SWOG 9346 has a similar design, except that the treatment comparison is being made in metastatic prostate cancer patients; these data are anxiously awaited.
Peripheral Androgen Blockade
The ability to block AR activity without depleting the body of testosterone led to the question of whether this “peripheral” blockade could be therapeutic in the absence of castration therapy. The side effect profile may be favorable, however the available data suggest that it may yield inferior disease control compared to castration or combined blockade. A Swedish study which compared bicalutamide 50 mg to orchidectomy in metastatic prostate cancer found that survival was significantly better in the castration group, HR 1.76 (95 % confidence interval 1.27–2.44) [26]. An Italian study which compared bicalutamide 150 mg to goserelin plus flutamide in advanced prostate cancer found no difference in progression-free or overall survival, although combined blockade trended towards superior results in the subgroup of metastatic patients [27]. Some have argued that the addition of 5-alpha reductase inhibitors more completely deprives cancer cells of androgen fuel, and yields deeper PSA nadir than bicalutamide monotherapy, comparable to that achieved with castration [28]. On the other hand, the 150 mg dose of bicalutamide has been associated with some safety concerns, such as a higher death rate when added to active surveillance in the early prostate cancer trialists group study [29], which has led the United States and Canada to recommend against prescribing the 150 mg dose [30]. Overall, at present, most physicians would not include peripheral androgen blockade as a standard first-line treatment option.
Up-Front Chemohormonal Therapy
No randomized studies to date have proven that the incorporation of chemotherapy prior to the emergence of castration resistance is beneficial. However, in vitro experiments show synergism between taxanes and androgen deprivation, and in mouse models combination therapy was associated with a doubling of time to disease progression compared to androgen deprivation followed by chemotherapy [31]. A European trial tested combined androgen blockade alone or with epirubicin and found that progression-free survival was significantly prolonged 12 months versus 18 months, p = 0.02, and overall survival showed a trend toward improvement 22 months versus 30 months, p = 0.1; in the subgroup of men with >5 bone metastases the survival difference approached significance, 17 months versus 27 months, p = 0.06 [32]. Phase II studies have shown promise for the addition of docetaxel to androgen deprivation therapy at biochemical recurrence [33], but the phase III studies which will definitively answer the question have not yet been reported.
Supportive Care for Men During Androgen Deprivation Therapy
Anticipating and minimizing the side effects of androgen deprivation as much as possible is a critical aspect of managing metastatic prostate cancer patients. In addition to ameliorating symptoms such as hot flashes, avoidance of serious metabolic and skeletal complications presents a significant challenge for urologists and medical oncologists. Side effects of androgen deprivation are summarized in Table 49.1 and depicted in Fig. 48.3.
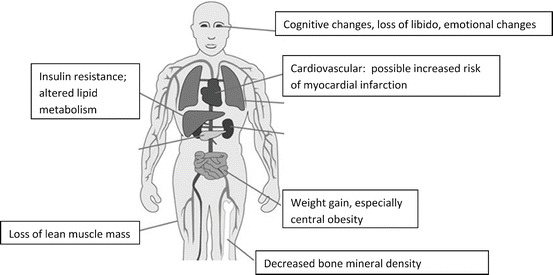
Table 48.1
Summary of adverse consequences of androgen deprivation and potential interventions to offset side effects
Adverse consequence | Possible interventions | References |
|---|---|---|
Bone mineral density loss | Exercise, vitamin D | Smith et al. [34] |
Toremifene | ||
Bisphosphonates | ||
Hyperlipidemia | Standard lipid medications | Levine et al. [38] |
Toremifene | Smith et al. [34] | |
Gynecomastia/mastodynia | Prophylactic breast irradiation | Dicker [39] |
Tamoxifen | Boccardo [27] | |
Hot flashes | Venlafaxine, gabapentin, pregalbin, clonidine, paroxetine, and fluoxetine | |
Acupuncture | Deng et al. [44] |

Fig. 48.3
An overview of some of the ways in which androgen deprivation affects organ function
Bone Mineral Density and Skeletal Related Events
One year of castration therapy is associated with the loss of 4 % of bone mineral density in the hip and 2 % in the spine [Diamond; 45] which corresponds to a fracture risk as high as 20 % at 10 years [35]. Bisphosphonates are effective in counteracting the bone mineral density loss; pamidronate administered every 12 weeks during a year of medical castration offset the bone mineral density loss induced by castration alone [46]. However quarterly administration, and even a single dose of zoledronic acid was subsequently shown to increase bone mineral density during a year of LHRH analogue therapy [36, 47]. Thus, bone mineral density should be evaluated at baseline and periodically during androgen deprivation so that appropriate patients requiring intervention can be identified. Calcium and vitamin D should be part of the approach to support bone mineral density, and weight-bearing exercise can also be recommended. The selective estrogen receptor modulator toremifene was also studied in this arena and was found to increase bone mineral density by 1.6 % in men receiving ADT compared to 0.7 % decrease in the placebo arm [47].
Bone metastases can cause significant morbidity, including pain, fracture, and spinal cord compression which are collectively labelled “skeletal related events”. Unravelling the unique crosstalk between prostate cancer cells and bone marrow stroma have facilitated the development and study of bone-targeted therapies. In castration-resistant metastatic disease, zoledronic acid administered intravenously every 3–4 weeks was shown to reduce the risk of skeletal related events from 44 to 33 %, including fractures, which were reduced from 22 to 13 % [49]. In addition, zoledronic acid was associated with a significant delay in the time to the first skeletal event, from 321 days to 488 days, p = 0.009 [50]. Denosumab, a monoclonal antibody against RANK ligand showed an even stronger reduction and delay in skeletal related events compared to zoledronic acid, with a median time to skeletal-related event of 17.1 months for zoledronic acid compared to 20.7 months for denosumab [51]. The benefits of these agents in castration-sensitive disease have not yet been well described; a CTSU trial C90202 is currently enrolling men with bony metastatic disease and randomizing them to early bisphosphonate therapy versus bisphosphonates at the time of castration resistance with a primary endpoint of skeletal related events. This will inform clinicians of the relative risks and benefits of starting therapy earlier; significant adverse events related to bone-targeted therapies in general include osteonecrosis of the jaw and for bisphosphonates there is also the potential for renal toxicity.
Alterations in Lipids
Increases in serum total cholesterol and triglycerides occur during androgen deprivation, though the degree and pattern of changes varies somewhat according to the form of ADT (ex: monotherapy or combined blockade). For instance, LHRH analogue therapy for 1 year has been associated with a 9 % increase in total cholesterol and 26 % increase in triglycerides [37]. Although this has been difficult to correlate with clinical outcomes such as myocardial infarction or cerebrovascular accident, the changes are concerning. Selective modulation of the estrogen receptor, for instance with toremifene, appears to ameliorate these changes, leading to an increase in HDL, decrease in total cholesterol as well as LDL and triglycerides [48].
Insulin Resistance and the Metabolic Syndrome
Androgen deprivation is known to induce insulin resistance, which appears to be a direct consequence of hypogonadism and independent of body mass index changes during ADT [52]. Using Medicare data, investigators documented an increased risk of developing outright diabetes after orchiectomy or LHRH analogue use, with hazard ratios of 1.34 and 1.44, respectively [53]. In addition, abdominal obesity and the metabolic syndrome are more prevalent in men on long-term ADT than in matched prostate cancer and normal controls (55 % versus 22 % and 20 %, respectively) [54]. Clearly this is a serious problem which warrants attention. Lifestyle counseling should be part of the treatment plan when androgen deprivation is employed, though specific interventions have not yet been prospectively evaluated.
Cardiovascular Morbidity
Alarm was raised when Medicare database analysis and review of radiation oncology clinical trial data suggested a significant increase in cardiovascular morbidity, namely myocardial infarction and sudden cardiac death, when men received androgen deprivation adjunctive to radiation [53, 55]. This is physiologically plausible, given the known propensity for androgen deprivation to cause weight gain, worsening lipids, and insulin resistance. However, other analyses have failed to find an association between ADT and cardiac mortality, or have found it limited to certain risk groups, such as those over 65 or with serious comorbidities [38]. As such there is no recommendation for routine involvement of cardiology in men undergoing ADT, but rather standard measures to control contributing risk factors such as lipids and blood pressure should apply. Undoubtedly this will continue to be an area of active research moving forward.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







