Tumour markers mandatory:
AFP: seen in 40–60 % nonseminomas and AFP is absent in seminoma; Even if raised in seminoma, the tumour is considered as mixed GCT
β-HCG: raised levels found in 10–15 % of stage I pure seminoma. Nearly 40–50 % NSGCTs have elevated levels of β-HCG
LDH: both SGCT and NSGCT show elevation of LDH.
Imaging:
Testicular ultrasound (7.5 MHz transducer)
CT-scan of abdomen and pelvis
Chest CT-scan (not mandatory for seminoma stage I)
Magnetic resonance imaging (MRI) of chest and abdomen: only if there is a contraindication for CT-scan (e.g. allergy to contrast media)
Bone scan: only if symptoms
Positron emission tomography (PET)-scan: to identify viable tissue in residual lesion ≥3 cm in advanced seminoma if determined ≥8 weeks after chemotherapy
Head CT may be required when indicated where cerebral metastasis are suspected.
Fertility investigations (should be offered):
Semen analysis; serum estimations of total testosterone, luteinising hormone (LH), follicle stimulating hormone (FSH), sperm banking (this may require screening tests for hepatititis and human immunodeficiency virus (HIV)
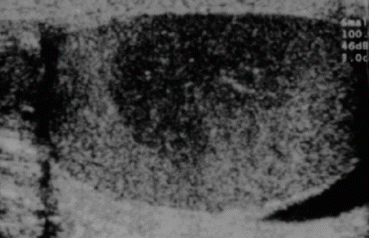
Fig. 28.1
Ultrasound of testis showing seminoma
Computerized Tomography (CT)
Contrast enhanced (oral and intravenous) CT of the chest, abdomen and pelvis is required as an initial staging investigation [5, 6]. For the evaluation of the lungs and mediastinum, chest CT scan is much more sensitive than a plain chest X-ray [7, 8]. However, it should be noted that pulmonary/pleural nodules of <1 cm can represent a false positive finding in CT scans [8]. Furthermore, CT scans of the abdomen and pelvis might give false-negative results in up to 30 % of cases due to difficulties in the interpretation of lymph nodes based on morphology and size alone [5]. Therefore, the differentiation between clinical stages I and IIA by CT might be unreliable. A detailed description of the location, number and size of lymph nodes should be provided in the radiology report. Magnetic resonance imaging (MRI) scans of the abdomen and pelvis do not provide additional information and should be restricted to those patients in whom intravenous contrast media is contraindicated [9]. Based on available data, PET has not conclusively demonstrated to improve sensitivity over CT staging alone [10, 11]. Even in high-risk stage I patients PET scan was not sensitive enough to predict early metastatic disease in a statistically significant proportion of patients. PET scans are therefore not recommended outside the clinical trials as a part of routine initial staging procedures.
Staging of Testicular Cancer
Clinical staging of a patient with a testicular germ cell tumour is done according to the TNM classification (Table 28.2) [12]. In order to verify clinical stage I disease markers need to be followed until normalization after orchidectomy. Those patients who do not have marker normalization after orchidectomy obviously have metastatic disease. Patients with metastatic disease are categorised according to the classification devised by the International Germ Cell Cancer Collaborative Group (IGCCCG) [13] which includes histology, location of primary tumour, location of metastases and pre-chemotherapy levels of AFP, HCG and LDH as prognostic markers to categorise patients into ‘good’, ‘intermediate’ and ‘poor’ prognosis groups (Table 28.3). The individual treatment strategy is based on both the TNM classification and the IGCCCG-prognostic factor-based classification.
Table 28.2
TNM Staging American Joint Committee on Cancer (AJCC) and Union Internationale Contre le Cancer (UICC) [12]
Primary tumour pT | ||||
pTX | Primary tumour cannot be assessed | |||
pT0 | No evidence of primary tumour (e.g. histological scar in testis | |||
pTis | Intratubular germ cell neoplasia (carcinoma in situ) | |||
pT1 | Tumour limited to testis and epididymis without vascular/lymphatic invasion: tumour may invade tunica albuginea but not tunica vaginalis | |||
pT2 | Tumour limited to testis and epididymis with vascular/lymphatic invasion, or tumour extending through tunica albuginea with involvement of tunica vaginalis | |||
pT3 | Tumour invades spermatic cord with or without vascular/lymphatic Invasion | |||
pT4 | Tumour invadas scrotum with or without vascular/lymphatic invasion | |||
Regional lymph nodes (N) | ||||
NX | Regional lymph nodes cannot be assessed | |||
N0 | No regional lymph node metastasis | |||
N1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension or multiple lymph nodes, none more than 2 cm in greatest dimension | |||
N2 | Metastasis with a lymph node mass more than 2 cm but not more than 5 cm in greatest dimension, or multiple lymph nodes, any one mass more than 2 cm but not more than 5 cm in greatest dimension | |||
N3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | |||
Pathologic (pN) | ||||
pNX | Regional lymph nodes cannot be assessed | |||
pN0 | No regional lymph node metastasis | |||
pN1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension and 5 or fewer positive nodes, none more than 2 cm in greatest dimension | |||
pN2 | Metastasis with a lymph node mass more than 2 cm but not more than 5 cm in greatest dimension; or more than 5 nodes positive, none more than 5 cm; or evidence or extranodal extension of tumour | |||
pN3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | |||
Distant metastasis (M) | ||||
MX | Distant metastasis cannot be assessed | |||
M0 | No distant metastasis | |||
M1 | Distant metastasis | |||
M1a | Non-regional lymph nodes(s) or lung | |||
M1b | Other sites | |||
Serum tumour markers (S) | ||||
Sx | Serum marker studies not available or not performed | |||
S0 | Serum marker study levels within normal limits | |||
LDH (U/l) | hCG (mlU/ml) | AFP (ng/ml) | ||
S1 | <1.5 × N and | <5,000 and | <1,000 | |
S2 | 1.5–10 × N or | 5,000–50,000 or | 1,000–10,000 | |
S3 | >10 × N or | >50,000 or | >10,000 | |
[N indicates the upper limit of normal for the LDH assay] | ||||
Except for pTis and pT4, where radical orchiectomy is not always necessary for classification purposes, the extent of the primary tumour in classified after radical orchiectomy; see pT. in other circumstances, TX is used if no radical orchiectomy has been performed. | ||||
Substages of stage I testicular cancer: | ||||
Stage IA | pT1 | NO | MO | S0 |
Stage IB | pT2, pT3 or pT4 | NO | MO | S0 |
Stage IS | Any pT/TX | NO | MO | S1-3 |
Table 28.3
IGCCCG prognostic grouping classification
Prognosis | 5-year-survival (%) | Non-seminoma | Seminoma |
|---|---|---|---|
Good | 90 | Testis or primary extragonadal retroperitoneal tumour | Any primary localisation |
And low markers | Any marker level | ||
AFP <1,000 ng/ml, | And no non-pulmonary visceral metastases | ||
And ß-HCG <1,000 ng/ml (<5,000 IU/l) | |||
And LDH <1.5× normal level | |||
And no non-pulmonary visceral metastases | |||
Intermediate | 75 | Testis or primary extragonadal retroperitoneal tumour | Any primary localisation |
And intermediate markers | And presence of non-pulmonary visceral metastases (liver, CNS, bone, interstitium) | ||
AFP 1,000–10,000 ng/ml | Any marker level | ||
And/or ß-HCG 1,000–10,000 ng/ml (5,000–50,000 IU/l) | |||
And/or LDH 1.5–10× normal level | |||
And no presence of non-pulmonary visceralmetastases | |||
Poor | 50 | Primary mediastinal germ cell tumour with or without testis or | – |
Primary retroperitoneal tumour | |||
And presence of non-pulmonary visceral metastases (liver, CNS, bone, intestinum) | |||
And/or “high markers” | |||
AFP >10,000 ng/ml, | |||
And/ or ß-HCG >10,000 ng/ml (50,000 IU/l) | |||
And/ or LDH >10× normal level. |
Spread of Testicular Tumor
The testicular tumors have a specific mode of spread, with the metastases occurring through lymphatic and vascular routes. Local spread is limited by the tunica albuginea of the testis. The lymphatic spread is a common form of metastasis in all histological types. Haematogenous spread occurs to the lungs, liver and brain. As testes develop in the retroperitoneum in the abdomen, their lymph drains to paraaortic and other lymph nodes in the infrarenal region. The lymphatics from the epididymis drain into testicular lymphatics. These lymphatics join lymphatics from the tunica albuginea in rete testis and proceed to the spermatic cord [14]. The lymphatics initially accompany testicular vessels. After crossing the ureter they spread out to join retroperitoneal lymph nodes anterior to the lumbar vessels [15, 16]. The right testicular vein drains into the anterior aspect of the inferior vena cava and the left testicular vein drains into left renal vein.
Right-sided tumors metastasize to the infrarenal aortocaval, precaval, right paracaval, and retrocaval lymph nodes. Left-sided tumors spread to the left paraaortic and preaortic lymph nodes. The echelon lymph nodes are lateral to the paracaval and paraaortic lymph nodes in the region between the first and third lumbar vertebra on the iliopsoas muscle, which can get involved particularly in cases with relapse [17]. When the anatomy of the inguinoscrotal region is disturbed by surgical procedures like orchidopexy or scrotal exploration, there is likely to be a direct spread to the iliac or inguinal lymph nodes, which normally drain scrotal skin.
Surgical Management
Radical Inguinal (High) Orchidectomy (RIO)
As a rule radical inguinal orchiectomy is a standard treatment and performed prior to any therapy [5]. This is performed through the inguinal approach with isolation the spermatic cord up to the level of internal inguinal ring followed by ligation/transfixation at that level and division of the cord. Testicular tumour markers are done before and repeated after the orchidectomy. The only exception to this rule is when patients present with a life-threatening metastatic disease and an unequivocally elevated AFP or β-hCG. In such cases, orchidectomy is done after the completion of chemotherapy [18–20]. The operation is performed through an inguinal incision. The tumour-bearing testicle is excised along with the spermatic cord to the level of the internal inguinal ring. In patients with negative serum tumour markers or small equivocal possibly benign tumours, histological analysis of a frozen section may be desirable prior to proceeding to orchiectomy or to allow organ sparing surgery, particularly if a benign tumour is found [21, 22].
Organ Preserving Surgery
In patients with synchronous bilateral tumours, metachronous contralateral tumours or solitary testicles with normal preoperative testosterone levels, organ-preserving surgery is an alternative procedure to orchiectomy and should be discussed with the patient. If organ preserving surgery is considered, the patient should always be treated at a tertiary referral centre with an experience in the management of testicular cancer [23–25]. When organ preserving surgery is performed, adjuvant radiotherapy is strongly recommended according to the management strategy for testicular intraepithelial neoplasia (TIN) in unilateral tumours [25]. Adjuvant radiotherapy may be delayed in patients who wish to father children. In patients who have non-obstructive azoospermia, sperm samples can also be retrieved at the time of surgery.
Fertility Preservation
All patients with established diagnosis of malignant testicular tumours should have pre-treatment option of semen preservation for future fertility treatment. It is also advisable to carry out an reproductive endocrine profile consisting of serum levels (Table 28.1) of follicle stimulating hormone (FSH), luteinising hormone (LH), testosterone (T) and semen analysis before orchidectomy [26]. The testis is easily affected by chemotherapy or radiotherapy and the effects are long-lasting and in some cases irreversible. Testicular toxicity affects spermatogenesis more than it affects testosterone production by Leydig cells [27]. The topic of fertility concerns may not be discussed at all by patients due to variety of factors: patients may be overwhelmed by the diagnosis of cancer diagnosis, they may be unaware that potential fertility loss could occur, or they may be concerned that pursuing fertility preservation might delay their cancer treatment leading to increased morbidity and mortality [28]. In such cases the onus is on the clinician to initiate the discussion on fertility preservation when it is applicable.
Diagnosis and Treatment of Testicular Intraepithelial Neoplasia (TIN) or Carcinoma in situ (Cis) or Intratubular Germ Cell Neoplasia (ITGCN)
The pathological aspect has already been discussed in detail in the pathology section. Nearly 70 % of all TIN will progress to an invasive GCT within a 7 years period if left untreated and followed by surveillance [29, 30]. ITGCN cells are typically located within the seminiferous tubules and usually there is no active spermatogenesis in the TIN bearing tubules (Fig. 28.2). These cells populate in numerous seminiferous tubules in the peritumoral parenchyma and basically all TGCT have at least some ITGCN cells in the adjacent parenchyma. They spread along the seminiferous tubules (Fig. 28.2) sometimes invading the rete testis; in some cases they may proliferate locally by filling a tubule by several layers (Fig. 28.3). As mentioned earlier, the cells are characterized by an overexpression of intracytoplasmic placental alkaline phophatase (PLAP) on immunohiostochemistry (IHC) (Fig. 28.3). According to recent recommendations, IHC for PLAP is mandatory for the adequate diagnosis of ITGCN [4, 31]. Besides ITGCN, only seminomas and embryonal carcinomas overexpress PLAP in similar abundance. About 10 % of ITGCN cells may not express PLAP at all and it might be helpful to stain with the 43–9 F [32].
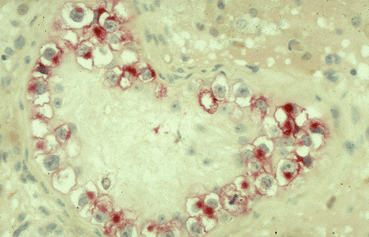
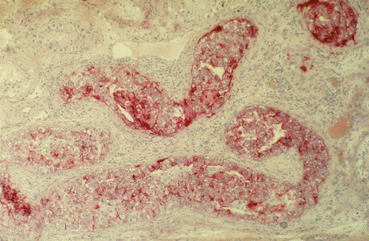

Fig. 28.2
Typical morphology of TIN cells: large cells with abundant glycogen, thickened basal membrane, no active spermatogenesis, overexpression of PLAP

Fig. 28.3
Multifocal spread of TIN populating numerous seminiferous tubules; TIN cells proliferate locally and fill the tubules in several layers
Currently, surgical testicular biopsy is the most reliable method for diagnosing ITGCN [33]. The biopsy should be taken in the craniolateral region of the testicle where the risk to damage intratesticular vessels is minimal. Damage to the specimen should be avoided. Dieckmann et al [33] in their prospective study of 2,318 testis cancer patients have shown that a two-site biopsy is significantly more sensitive to detect ITGCN than a single site biopsy [33]. A total of 119 (5.1 %) exhibited ITGCN in contralateral testis with a discordance rate of 31 % of the biopsies. The discordance was more frequent in patients with normal testicular volume and unimpaired spermatogenesis.
A critical analysis of the available data on contralateral testicular biopsy in patients with untreated testicular cancer does not support the idea of routine testis biopsy in all cases [34, 35]. Contralateral testis biopsy might be offered to high risk patients (testis volume <12 ml, history of cryptorchidism, age <30 years, table 28.4) [35], however, this procedure does not result in improved survival rates nor in decreased treatment associated toxicity in secondary testicular germ cell tumors.
Table 28.4
Clinical risk factors for contralateral TIN in patients with unilateral testicular germ cell tumors
Risk factor | Relative risk | 95 % confidence interval |
|---|---|---|
Testicular atrophy (<12 ml) | 4.3 | 2.83–6.44 |
History of cryptorchidism | 2.1 | 1.21–3.63 |
Age <30 years | 1.7 | 1.17–2.6 |
Family history of testis cancer | 2.2 | 1.25–12.3 |
Infertility | 1.6 | 1.10–10 |
Management of Seminoma
The role of surgery in pure seminomas is limited mostly to the radical orchidectomy. Post-orchidectomy management for stage I disease involves surveillance, with treatment reserved for patients who have relapse, or adjuvant treatment with either radiotherapy or chemotherapy [36] and this aspect of management is described later. Retroperitoneal lymph node dissection is not recommended.
Primary RPLND is not an option stage I seminoma even after relapse and for stage II seminomas; RPLND has been replaced largely by chemotherapy and/or radiotherapy [37]. In a retrospective study comparing radiotherapy and RPLND in stage I and II seminoma, Warszawski et al [38] observed in their 161 subjects treated between 1975 and 1991 (98 patients received radiotherapy and 63 patients underwent RPLND) a higher relapse rates after RPLND (9.5 % Vs. 2.0 %).
Post-chemo RPLND (PC-RPLND) in seminomas: The original recommendation was to resect tumours >3 cm diameter. However viable seminoma is found in less than 20 % of patients after chemotherapy. Also pure seminoma is a different entity compared to NSGCT. There is a higher degree of desmoplastic reaction than NSGCT and there is relatively higher grade of poor tissue planes. Most often the excision of residual masses cannot be completed in a safe manner with nearly 40 % of patients requiring additional operative procedures [39]. Overall RPLND does not seem to have any therapeutic benefit even when undertaken as a technically challenging procedure.
Low Stage NSGCT
Clinical Stage I
Definition
Clinical stage I is defined by negative imaging studies of the chest, the abdomen and the pelvis. Furthermore, in order to verify clinical stage I disease elevated markers should be followed up in the post-orchidectomy period until normalization. Patients who fail to normalize after orchidectomy or those in whom markers do not decline according to their half life after orchidectomy do not have stage I disease.
The standard treatment options of patients with clinical stage I disease remains controversial since patients have an excellent survival rates with all forms of treatment including retroperitoneal lymph node dissection (RPLND), active surveillance and primary chemotherapy [4]. The controversy has remained over the last two decades as nearly 30 % of these patients would harbour occult microscopic retroperitoneal lymph node metastases, which cannot be reliably detected by modern imaging studies, tumour markers or molecular approaches. With RPLND the staging process becomes more reliabe and accurate; however, nearly 70 % are operated unnecessarily and 10 % will develop systemic metastases with a need for salvage chemotherapy. With primary chemotherapy alone approximately 50–70 % of the patients are overtreated and might be exposed to unnecessary long-term complications. Active surveillance on the other hand is clearly indicated in low risk disease which has a recurrence rate of only 15 %. In patients with a high risk disease the relapse rates vary between 35 and 55 % and makes intensive salvage chemotherapy necessary.
Prognostic Risk Factors
Lymphovascular infiltration (vascular invasion, VI) by the primary tumour is one of the most important prognostic indicators for occult metastases and must be assessed in all patients [40–44]. Without adjuvant treatment 48 % of the patients with VI would develop metastases while only 14–22 % of those without metastasis relapse. Based on these data, VI alone does not represent a valuable prognostic risk factor for a risk-adapted approach as it is likely to result in an unnecessary overtreatment rate of about 50 %. The proliferation rate and the percentage of embryonal carcinoma (EC) present in relation to the total tumour volume are further prognostic indicators [42, 43]. The combination of absence of VI and a percentage of EC <45 % correctly identified 91.5 % of all patients with true pathological stage I disease [42]. On the other hand, the presence of VI and a percentage of EC >80 % correctly predicted pathological stage IIA/B disease in 88 % of the patients. Based on these data, the German Testicular Cancer Study Group performed a prospective study in which 200 patients with clinical stage I NSGCT were assigned to RPLND and risk factors were assessed prospectively [43]. The combination of absence of VI, percentage of EC <50 % and a MIB-1 proliferating index <20 % correctly identified pathological stage II disease with 86.5 % accuracy. If none of the prognostic risk factors were present, the risk of occult retroperitoneal disease was 16 % and patients were classified as low risk. The risk of lymph node metastases was 65 % if at least VI and percentage EC >50 % were present and the patients were classified high risk. In another small prospective evaluation, Perotti et al [44] tested a prediction model in which patients with a percentage of EC >80 % and/or the presence of VI were assigned to a high risk of occult metastatic disease. The authors correctly predicted final pathological stage II disease in 67 % when only one prognostic factor was present.
The combination of imaging studies, histopathological evaluation and immunhistochemical techniques is likely to improve the prediction of the final pathological stage of the disease. Localization and size of lymph nodes in conjunction with a low volume of embryonal carcinoma, absence of vascular invasion and a low MIB-1 proliferation rate might give important information with regard to the probability of lymph node metastases. In a retrospective analysis of 91 clinical stage 1 NSGCT patients using a combined approach with quantitative immunohistochemical, histopathologic, and radiologic assessment who underwent nerve-sparing RPLND, Liebovitch et al [45] found 40 out of 41 patients were correctly classified as low risk tumours for metastases. They concluded Patients with lymph nodes <1 cm diameter which are located in the primary landing zone, a low volume of embryonal carcinoma harbour a risk of <10 % of occult retroperitoneal lymph node metastases and might be best managed by active surveillance.
Treatment of Patients with Non-seminoma Clinical Stage I NSGCT
If the correct treatment were to be executed, the cure rate for patients with CS I NSGCT should be 99 % regardless of the treatment chosen. Basically, three treatment options, which have similar cure rates but significantly differing in frequency and type of treatment-associated toxicities, might be offered to these patients: (i) active surveillance; (ii)primary chemotherapy with 1–2 cycles PEB and (iii) Nerve-sparing RPLND. When choosing a risk–adapted approach in clinical stage I NSGCT, the clinician has to reflect that all the patients are expected to be long-term survivors and there that long-term side effects of treatment would be minimal or non existent. It is therefore the aim of ongoing clinical research to minimize the modalities of treatment and their toxicities without compromising therapeutic efficacy. The European Germ Cell Cancer Consensus Group Conference (EGCCCG) recommends low risk patients that they should be primarily offered active surveillance [4], whereas systemic chemotherapy with 2 cycles PEB represents the treatment of choice for high risk patients.
Active Surveillance
Active surveillance encompasses a treatment strategy with the aim of detecting retroperitoneal or systemic relapses and to treat only those patients with documented metastatic disease thereby decreasing the risk of unnecessary overtreatment. During the initial post-treatment phases of follow-up, regular clinical examinations, monitoring of serum tumour markers and imaging investigations are carried out. The frequency and type of examinations are dependent on the estimated risk of relapse and the time that has elapsed since completion of therapy and should be modified according to these risks. However, only limited information about the optimal follow-up strategy exists.
When recommending active surveillance for low risk or in certain scenarios also for stage I high risk NSGCT, two major important aspects have to be considered: (1) risk of secondary malignancies due to the repetitive radiation exposure of the imaging studies and (2) more intensive treatment in case of relapse (3 cycles PEB ± postchemotherapy RPLND) as compared to primary active therapy (1 cycle PEB).
In patients who were on active surveillance for CS I with a low risk NSGCT, the relapse rate was 27–30 % on a long-term follow up of ≥20 years [41, 46, 47]. Relapses occur in the retroperitoneum in 54–78 % of patients, in the lungs in 13–31 %, but are rarely seen in more than one visceral organ. With this approach 78–86 % of patients do not need any further treatment after orchiectomy [41, 43, 46–48]. If a patient under surveillance relapses, the administration of chemotherapy will result in a cure rate close to 100 %. When the surveillance is not suitable, adjuvant chemotherapy with two cycles of BEP is recommended. Nerve sparing retroperitoneal lymph node dissection (RPLND) is a feasible option at high-volume centers [43]. A randomized phase III trial of one cycle BEP versus RPLND in 382 unstratified patients with clinical stage I disease (plus adjuvant chemotherapy for those who were to be pathological stage II after RPLND) suggested a significantly reduced recurrence rate using adjuvant BEP as compared to surgery (1.1 % versus 7.5 %, respectively [43].
Patients with a low risk of relapse (no VI) for follow up should be managed by surveillance according to the EGCCCG recommendation. This requires at least 5 CT scans performed at 0, 3, 12, 18 and 24 months [4]. This follow-up protocol with extensive imaging studies, however, might lead to a high radiation exposure with significant long-term consequences for the patients.
In a recent study, Tarin et al. [49] estimated the risk of secondary cancer associated with CT imaging related radiation during the surveillance of stage I NSGCT patients. In their analysis they evaluated surveillance protocols recommending about 16 CT scans over a 5-year period and they took into consideration a 64-slice CT scanner obtaining images of the abdomen and pelvis with and without inclusion of the chest. For calculation of organ specific radiation doses a standardized, phantom male patient was employed using the Monte Carlo simulation techniques. With a 5-year surveillance protocol the lifetime cancer risk ranged from 1 in 52 (1.9 %) for an 18-year old to 1 in 63 (1.2 %) for a 40-year old patient. If chest CTs were also obtained the risk increases to 1 in 39 (2.6 %) and 1 in 58 (1.6 %), respectively. The relative risk of a secondary malignancy with surveillance compared to a single scan after RPLND is approximately 15.2.
Various studies have been designed to reduce the number of CT scans during the surveillance strategy. Atsü et al [50] analysed the outcome of 140 CS I NSGCT who were followed with only 2 CT scans at postoperative months 6 and 12 with no CT scans thereafter. All patients underwent serial measurements of the serum tumour markers, abdominal ultrasonography and chest X-rays at variable frequency depending on the time intervals between orchidectomy and follow-up. Relapses developed in 32 (24 %) patients and they were detected within a median of 5 (2–23) months. 28 relapses developed during the first year and only 4 relapses occurred during the second year of surveillance. All patients were salvaged by systemic chemotherapy combined with postchemotherapy RPLND in 7 cases. In their study, the presence of any EC in the orchiectomy specimen resulted in a 3.7-fold increase of the relapse risk. In order to reduce the number of CT scans during follow-up, the prospective randomized Medical Research Council Trial (MRC, UK) TE08 was initiated which compared the diagnostic efficacy of 2 versus 5 CT scans during the first 2 years of follow-up to detect the number of patients who relapse with intermediate and poor prognosis disease at relapse [51]. 247 patients and 167 patients were randomized to the two-scan and the five-scan group, respectively. Besides CT scans all patients underwent follow-up assessments at various time intervals: clinical examination, evaluation of serum tumour markers AFP, ß-hCG and LDH as well as chest X-ray With a median follow-up of 40 months, 37 (15 %) relapses had developed in the two-scan and 33 (20 %) relapses occurred in the five-scan group. None of the patients had a poor prognosis disease at the time of relapse, but 2 (0.8 %) patients and 1 (0.6 %) patient had intermediate prognosis disease. There were, however, some other statistically significant differences between the two groups with regard to the indicators of relapse. The proportion of patients in whom elevated tumour markers were the first indicators of relapsing disease was 21.6 and 6.1 % in the two-scan and the five-scan group, respectively. Interestingly, 16 patients had normal markers at time of their orchidectomy but elevated markers at time of relapse underlining the importance of serum tumour markers measurements in every patient with CS I NSGCT who undergoes active surveillance. In the two arms combined a total of 11 patients developed lung metastases with 7 of them being tumour marker negative. The following conclusions can be drawn from this large prospective randomized trial: (a) fewer CT scans reduce the radiation exposure and costs without harming the patient, (b) regular measurements of tumour markers ß-hCG, AFP and LDH together with chest X-ray and two abdominal CT scans are necessary for a surveillance program, and (c) it is unclear if this approach of reduced imaging studies can be applied for high risk patients since only 10 % of the recruited NSGCT demonstrated vascular invasion with a relapse rate of 32 %.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







