Chapter 99 Techniques of liver replacement
Historical Overview
The steps by which liver replacement became the treatment of choice for numerous end-stage liver diseases (Starzl et al, 1989) were summarized in 2002 (Starzl). The basic operation was developed in dogs during the years 1958 through 1960 and was attempted clinically in 1963 under azathioprine-prednisone immunosuppression. The first humans to have liver replacement with prolonged survivals were reported by Starzl in 1969; however, not until the availability of cyclosporine in the 1980s did orthotopic liver transplantation became accepted worldwide as effective therapy. The results improved again with the advent of tacrolimus in the 1990s (see Chapter 96).
Elements other than immunosuppression have contributed to the success of liver replacement, including improved patient selection and pretransplantation management, noninvasive diagnostic techniques, new antibiotics, and advances in anesthetic and perioperative critical care (see Chapters 97A and 97B); however, perfection of the donor and recipient operations was the crucial factor on which all else ultimately depended. Surgical techniques used at the University of Colorado—and since January 1981, at the University of Pittsburgh—are presented in this chapter, with an emphasis on principles rather than details.
Donor Operation
The use of multiple organs from a single cadaveric donor became practical with the development of standard procurement methods in the early 1980s (see Chapter 98A). Subsequently, the University of Wisconsin (UW) and histidine-tryptophan-ketoglutarate (HTK) preservation solutions made storage of hepatic grafts relatively safe for 12 to 18 hours. The availability of this much time has allowed widespread sharing of livers while permitting an accurate assessment of the grafts by histologic and metabolic criteria.
Standard Liver Procurement
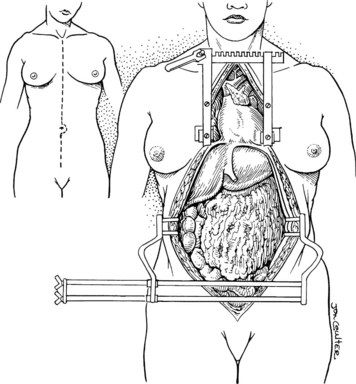
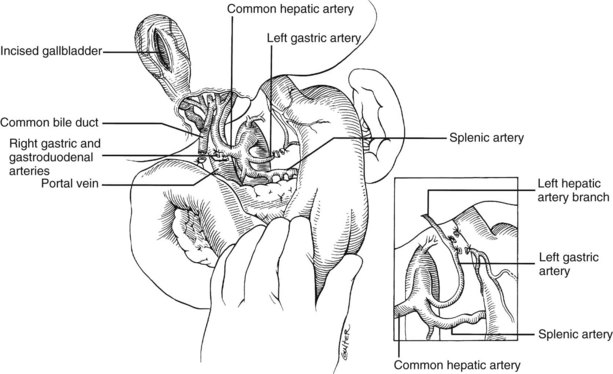
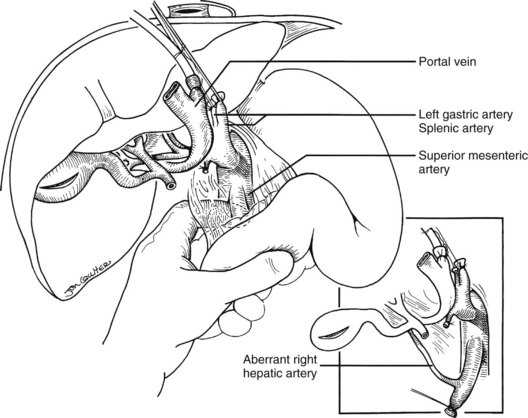
In the standard procurement technique, a midline incision is made from the suprasternal notch to the pubis to expose the abdominal and thoracic organs of potential interest (Fig. 99.1). After verification that the liver has a normal consistency and color, the left suspensory ligament is incised, allowing the left lobe to be retracted anteriorly and to the right. This retraction exposes the upper part of the gastrohepatic ligament, which contains the left gastric artery, the smallest branch of the celiac axis, and the arterial supply of the liver (Fig. 99.2). If an anomalous left hepatic arterial branch originates from the left gastric artery (Fig. 99.3), it must be preserved in continuity with the main left gastric artery (see Fig. 99.3, inset). When present, this anomalous left hepatic arterial branch is nearly always present just posterior to the vagus nerve branch, as it courses from the lesser curvature of the stomach through the gastrohepatic ligament to the liver.
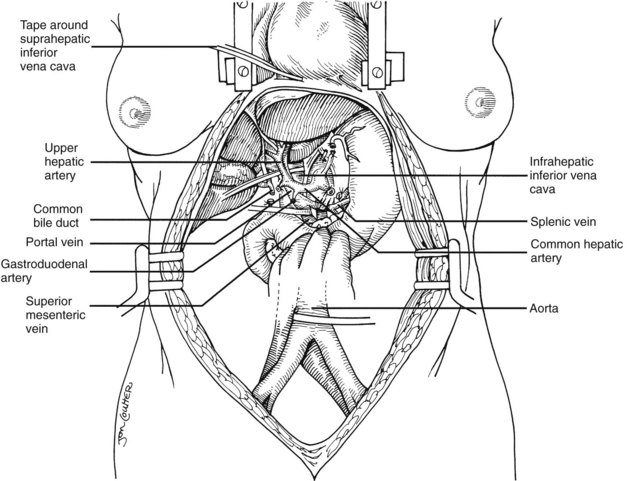
The largest branch of the celiac axis usually is the common hepatic artery. The right gastric and gastroduodenal arteries are ligated and divided (Fig. 99.4; see also 99.2). If the left gastric, right gastric, and gastroduodenal arteries are ligated in that order, the subsequent dissection of the common duct and the portal vein is rendered relatively bloodless. The common bile duct is transected near the duodenum, and the gallbladder is incised, permitting the bile to be irrigated out with saline (see Fig. 99.3); this avoids autolysis of the extrahepatic and intrahepatic bile duct epithelium during storage. The portal vein now is dissected inferiorly to the confluence of the splenic and superior mesenteric veins (see Fig. 99.4).
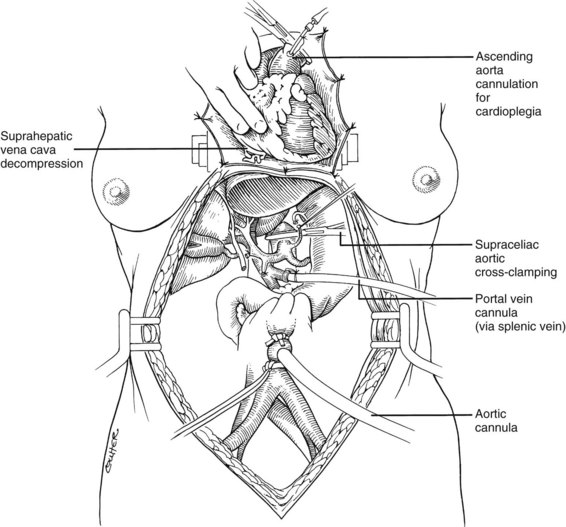
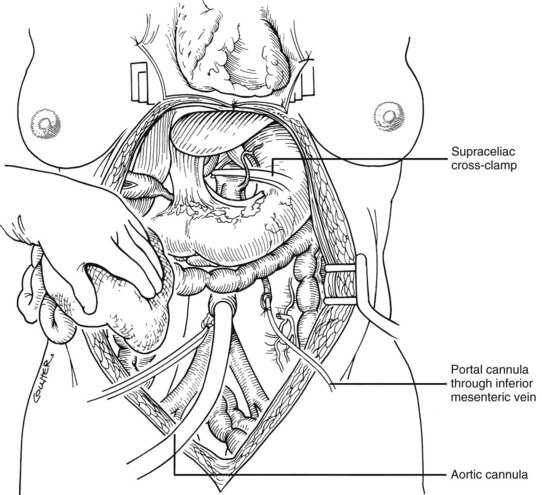
After completing the hilar dissection, the aorta is encircled superiorly, where it passes through the diaphragm, and inferiorly, just proximal to its distal bifurcation. Cannulae for infusion are placed into the inferior mesenteric vein (or splenic vein) and, after total body heparinization, into the distal aorta (see Fig. 99.4). When all procurement teams are ready, the aorta is cross-clamped at the diaphragm or in the chest by the abdominal surgeon (see Fig. 99.4), while the thoracic surgical team clamps the ascending aorta. Moderately rapid infusion of cold preservation solution is started into the portal circulation and aortic cannula. At the same time, a cardioplegia solution is infused into the midportion of the ascending aorta. Congestion of the various organs is prevented by an incision in the suprahepatic inferior vena cava at the level of the right atrium, which allows the blood and infusate to drain into the pericardium (see Fig. 99.4).
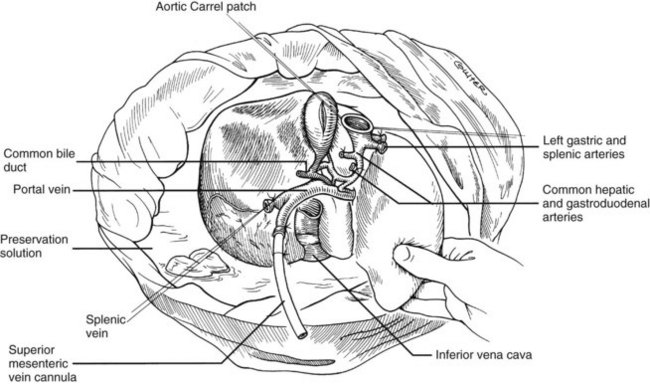
In adults, the liver is usually perfused with 2 L of HTK infused through the splenic vein or inferior mesenteric vein and 10 L infused through the aorta, although smaller volumes are used for children. When the liver becomes cold and blanched, and the heart has been removed, the total hepatectomy is completed. The remaining dissection must be performed expeditiously but methodically. If the celiac axis is retained with the graft, a proximal segment of its splenic arterial branch also should be conserved for potential reconstruction of an anomalous hepatic artery (see later). The most common hepatic artery anomaly is an aberrant right hepatic artery that originates from the superior mesenteric artery, commonly found posterior to the portal vein (Fig. 99.5; see Chapter 1B). If the pancreas is to be discarded, the anomalous retroportal artery can be kept in continuity with the superior mesenteric artery (see Fig. 99.5, inset), where its origin can be incorporated into a Carrel patch that is shared with the origin of the celiac axis.
The liver now remains attached primarily by the vena cava above and below the liver. The vena cava below the liver is transected above the entry of the left and right renal veins (Fig. 99.6). The vena cava above the liver is transected with a surrounding rim of diaphragm that is carefully excised on the back table. The retrohepatic vena cava is dissected free, including ligation of the right adrenal vein and posterior lumbar tributaries. The liberated liver is immediately placed in a solution-filled preservation bag packed in ice (Fig. 99.7); some surgeons flush the common bile duct with HTK before packaging the liver.
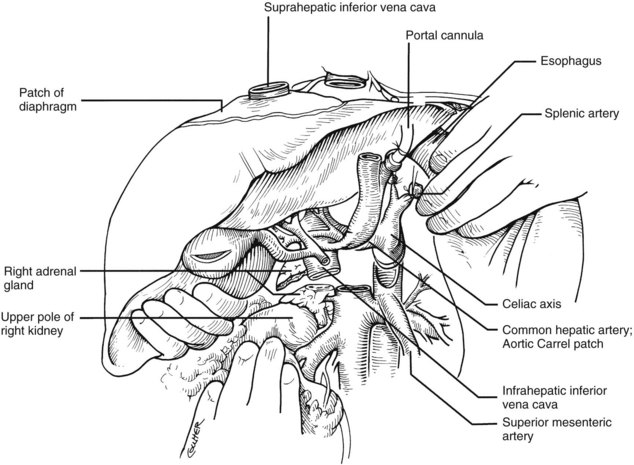
FIGURE 99.6 The suprahepatic vena cava has been transected with inclusion of a generous patch of diaphragm on the liver side. The infrahepatic vena cava is divided just above the origin of the renal veins, and the celiac axis is removed with a Carrel patch of anterior aorta. If an anomalous right hepatic artery originates from the superior mesenteric artery (SMA), the origin of the SMA may be included in the Carrel patch (see Fig. 99.10A).
Modified Donor Procedures
Rapid Procurement
Use of the standard technique in stable donors has allowed the training of relatively inexperienced surgeons in the performance of a donor hepatectomy. When the technique is mastered, faster methods can be applied electively or, if required, by urgent clinical circumstances. With the rapid techniques, little or no preliminary dissection is done except for encirclement of the supraceliac aorta and cannulation of the inferior mesenteric vein and terminal aorta (Fig. 99.8). If the heart is to be removed, the cardiac surgeon proceeds as if other organs are not to be harvested but gives warning before the circulation is stopped.
At the moment heart function ceases, the abdominal aorta is cross-clamped above or just below the diaphragm, and an infusion of cold HTK solution is started in the inferior mesenteric vein and distal aorta (see Fig. 99.8). The amount of preservation fluid with the rapid technique is approximately the same as that used for the standard method (i.e., 2 L into the inferior mesenteric vein (IMV)/splenic vein and 10 L through the aorta). When the liver becomes cold, the infusions are slowed. In the now bloodless field, the main vessels of the celiac axis can be quickly dissected, and the hilar dissection can be completed in a matter of minutes.
Super-Rapid Procurement
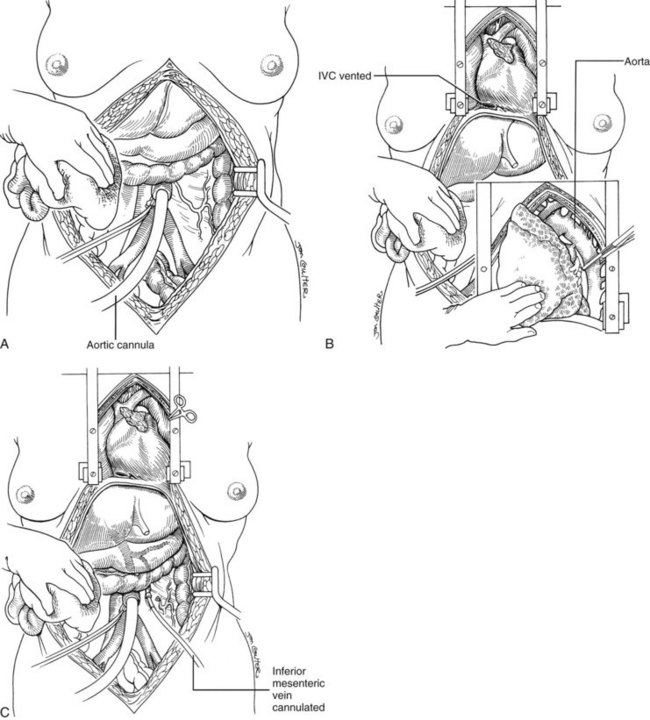
In arrested or non–heart-beating donors, an even quicker procedure can be used to procure satisfactory organs. This method also can be applied in countries that do not have “brain death” laws or under special legal or religious circumstances. Here, cooling requires urgent cannulation and cold fluid infusion into the distal aorta (Fig. 99.9A). Sternum splitting, thoracic aortic cross-clamping, and severance of the suprahepatic inferior vena cava for venous decompression are performed (Fig. 99.9B), deferring cannulation and perfusion of the portal venous system until after the various organs are at least partly cooled intraarterially (Fig. 99.9C). The various dissections are done in the same way as with the standard and rapid techniques. Effective application of this method requires an extremely high level of skill.