Prostate cancer is the most common male cancer and one of the top causes of male cancer-related death in Western countries. Most patients with prostate cancer respond to initial androgen deprivation therapy but eventually progress to castration-resistant prostate cancer (CRPC). Although androgen receptor signaling remains the main driver in CRPC, a growing body of evidence suggests that other pathways are involved in this progression. This article reviews the preclinical data and current status of clinical trials therapeutically targeting tubulin, DNA repair, molecular chaperones such as CLU and Hsp27, tyrosine kinases, and DNA repair.
- •
Many pathways are involved in cell survival or bone metastases, in addition to the AR pathway in CRPC.
- •
Tubulin-targeting agents are still under clinical investigation.
- •
Preclinical studies support DNA repair targeting in CRPC.
- •
Cell survival pathways are critical for resistance to endocrine therapies and chemotherapy in prostate cancer.
- •
MET and Src pathways are involved in many cellular processes leading to metastasis formation in prostate cancer, supporting their inhibition.
Introduction
Prostate Cancer (PCa) is the most common cancer in North America and the second leading cause of cancer-related death in men. Despite improved outcomes through early detection and treatment of localized PCa, many men still die of metastatic disease. Although androgen ablation remains the most effective management option for patients with advanced disease, most progress to castration-resistant prostate cancer (CRPC) within 2 years of treatment initiation. CRPC progression is a complex process by which cells acquire the ability to survive and proliferate in the absence of testicular androgens. Many mechanisms have been postulated to account for androgen receptor (AR) activation in CRPC tumors, including (1) activation of AR by nonsteroids such as growth factors and cytokines via deregulated multiple signaling pathways; (2) genetic mutation(s) or amplification(s) of AR that render the receptor hyperactive, which sensitizes cells toward low levels of androgen; (3) altered expression of activity of AR coactivators or chaperone proteins; (4) expression of AR splice variants that lack the ligand-binding domain (LBD) and are constitutively active in a ligand-independent manner; and (5) intratumoral steroidogenesis. These proposed mechanisms are not mutually exclusive and they likely work in concert to drive CRPC. Despite the failure of maximal androgen blockade trials using first-generation nonsteroidal AR inhibitors like flutamide or bicalutamide, CRPC tumors are not uniformly hormone refractory and remain sensitive to therapies directed against the AR axis. Hence, several new classes of AR-targeting agents are now in clinical development, including inhibitors of steroidogenesis (abiraterone) and more potent AR antagonists (MDV3100). Although enthusiasm for this approach remains high, prostate tumor heterogeneity and adaptive responses that support development of resistance via alternative mechanisms create a critical need for other strategies to kill prostate cancer cells.
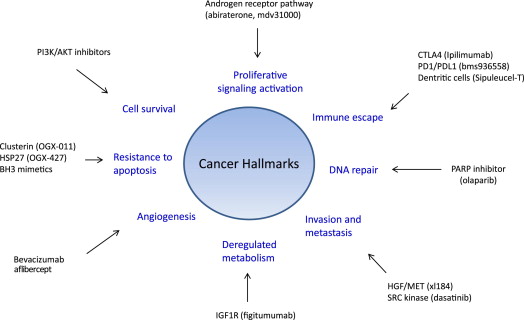
Improved understanding of prostate cancer biology is facilitating the identification of many pathways, aside from the AR, that drive CRPC progression by promoting invasion and metastasis, or by activating cell survival signaling. This article highlights the implications for practice, and focuses on those mediating cell survival, cell invasion, and cell proliferation ( Fig. 1 ).
Targeting cell proliferation
New Taxanes: Cabazitaxel
Preclinical data
Microtubules consist of tubulin heterodimers and radiate from the centrosome in the cytoplasm of interphase cells. They are involved in many cellular functions including cell motility, cell division, and intracellular transport. During the mitosis, microtubule networks ensure the correct attachment and segregation of chromosomes during cell division, making them attractive targets for anticancer drugs. Docetaxel is a taxane that binds to β-tubulin of microtubules during the G 2 -M phase of the cell cycle, leading to cell apoptosis in cycling cells. Recent studies have also shown additional effects of docetaxel on AR by preventing its translocation into the nucleus and thus inhibiting AR-induced signaling. Docetaxel-based chemotherapy is standard first-line chemotherapy in patients with metastatic CRPC (mCRPC). Two randomized phase III trials have shown an improved overall survival in chemonaive patients with CRPC treated with docetaxel alone or in combination with estramustine compared with mitoxantrone. However, almost 50% of patients do not respond to docetaxel.
Molecular mechanisms of resistance to docetaxel are multifactorial and involve decreased cellular drug accumulation caused by overexpression of membrane-bound efflux proteins (P-glycoprotein), expression of tubulin isotypes (overexpression of β-III tubulin) and defects in apoptosis. Some gene expression signatures have been associated with docetaxel resistance of prostate cancer cells, but no single driver pathway that responds to drugs has emerged to target and circumvent such resistance. New taxoid compounds have been developed as a low-affinity substrate for the P-glycoprotein efflux pump mechanism. Cabazitaxel (XRP6258, Jevtana) is a novel tubulin-binding taxane drug with antitumor activity in docetaxel-resistant cancers. Cabazitaxel promotes the assembly of tubulin and stabilizes microtubules against cold-induced depolymerization in vitro as potently as docetaxel. Cabazitaxel showed potent antitumor activity comparable with docetaxel in docetaxel-sensitive cell lines and exhibited more potent cytotoxic activity than docetaxel in cancer cell lines with acquired resistance to docetaxel caused by P-glycoprotein overexpression. Cabazitaxel resulted in antitumor activity on a broad array of tumor xenografts.
Clinical data
Phase 1 and 2 clinical studies reported that neutropenia is the primary dose-limiting toxicity, a recommended phase 2 dose between 20 and 25 mg/m 2 , and antitumor activity in solid tumors including docetaxel-refractory mCRPC, supporting further clinical investigations. Given the precedent efficacy of docetaxel in CRPC, along with the safety profile and observed responses in the phase I trial, an international phase III trial was conducted without phase II in patients with CRPC. The TROPIC trial was an international randomized phase 3 trial assessing cabazitaxel in men with mCRPC whose disease had progressed during or after treatment with a docetaxel-based regimen. Patients were randomly assigned to receive either cabazitaxel (25 mg mg/m 2 intravenously every 3 weeks) or mitoxantrone (12 mg/m 2 every 3 weeks). The primary end point was the overall survival (OS). This study showed that cabazitaxel improved median OS by 2.4 months (15.1 months vs 12.7 months) compared with the mitoxantrone group, with a hazard ratio (HR) reduction of 0.70 (95% confidence interval [CI] 0.59–0.83, P <.0001). Median progression-free survival (PFS) was also better in the cabazitaxel group (2.8 months, 95% CI 2.4–3.0) in the cabazitaxel group and 1.4 months (1.4–1.7 months) in the mitoxantrone group (HR 0.74, 0.64–0.86, P <.0001). The most common clinically significant grade 3 or higher adverse events were neutropenia (cabazitaxel 82% of patients vs mitoxantrone 58%) and diarrhea (6% vs <1%). A phase III trial is currently comparing cabazitaxel with docetaxel in chemonaive patients with mCRPC.
Epothilones
Preclinical data
Epothilones are, like taxanes, tubulin-binding agents but have different binding sites on the tubulin polymers. They induce apoptosis in cancer cells by disrupting the dynamic characteristics of microtubules. The epothilones include natural epothilone B (EPO906; patupilone) and several semisynthetic epothilone compounds such as BMS-247550 (ixabepilone) and sagopilone (ZK-EPO). Epothilones have cytotoxic effects against human cancer cells with high expression of P-glycoprotein and are also active in cancer cells resistant to taxanes because of tubulin mutations. Studies have shown that the precise binding sites and mode of action of epothilones differ from taxanes ; for example, ixabepilone can bind multiple β-tubulin isoforms, suppressing the dynamic instability of class III β-tubulin and class II β-tubulin microtubules, whereas taxanes weakly link class III β-tubulin. Epothilones also have low susceptibility to classic taxane resistance mechanisms, such as P-glycoprotein or multidrug resistance protein efflux, tubulin mutations, and alterations in tubulin isotypes.
Epothilone B (patupilone) has tumor activity in human prostate cancer DU-145 and PC-3 xenografts. Patupilone has antitumor activity against both paclitaxel-sensitive and paclitaxel-refractory cancers in vitro including in human cancer cell lines with P-glycoprotein overexpression and human cancer cell lines with tubulin mutations. Like epothilone B, ixabepilone has potent antitumor activity in mouse xenograft models bearing paclitaxel-sensitive or paclitaxel-refractory tumors. However, published preclinical data in prostate cancer xenografts are currently limited. Many studies have reported that ixabepilone has a low susceptibility to mechanisms of resistance to taxanes such as tubulin mutations and overexpression of the efflux pumps.
Sagopilone is a fully synthetic formulation of epothilone B. In vivo, sagopilone has shown activity in many cancers including prostate cancer, and cancer models with an multidrug-resistant phenotype and paclitaxel-resistant cells.
Clinical data
Phase I clinical trials indicate that epothilone and ixabepilone are well tolerated in patients who have cancer both as a single agent or in combination. However, neuropathy has led to frequent dose reductions in patients treated with ixabepilone in phase I clinical trials. The phase II clinical trials assessing ixabepilone suggest antitumor activity in men with mCRPC as single agent or in combination with estramustine. These agents have shown better activity in men with chemotherapy-naive mCRPC than in men previously treated with docetaxel. A phase II randomized study compared ixabepilone 35 mg/m 2 every 3 weeks with intravenous mitoxantrone 14 mg/m 2 every 3 weeks plus prednisone 5 mg twice daily in 82 patients with taxane-refractory CRPC. The efficacy end points were not different between the 2 arms. However, another phase II study showed that the combination of ixabepilone and mitoxantrone is both feasible and active in CRPC.
A phase II trial of patupilone (8 mg/m 2 ) was safe, had antitumor activity, and was associated with symptomatic improvement in patients previously treated with docetaxel. A prostate-specific antigen (PSA) decline of greater than or equal to 50% occurred in 47% of patients, whereas a partial measurable disease response occurred in 24% of assessable patients. A patient-reported pain response was also observed in 59% of assessable patients. In a recent phase II study, the activity of sagopilone 16 mg/m 2 given intravenously over 3 hours every 21 days was investigated in chemotherapy-naive patients with mCRPC in combination with oral prednisone. Of the 24 patients evaluable for response, 5 (21%) achieved a PSA response (reduction ≥50%) and 14 (58%) had a 30% PSA reduction within 3 months of enrollment. Among the 12 patients with measurable disease, there was 1 confirmed complete response (CR) and 1 confirmed partial response (PR), and 4 further patients achieved unconfirmed PRs.
Third-generation taxanes such as TPI 287 and agents targeting the mitotic process (ispinesib targets kinesin spindle protein, and danusertib targets the serine-threonine aurora kinase) are currently investigated in phase II clinical trials in men with mCRPC previously treated with docetaxel.
DNA Repair Inhibition
Preclinical data
DNA repair is essential because DNA is susceptible to spontaneous damage. Cellular DNA is susceptible to carcinogens, and the target of a broad range of anticancer agents. Several cancer susceptibility genes therefore encode for DNA repair and DNA damage response factors. DNA repair is coupled with DNA damage responses that are commonly referred to as checkpoint responses. Those checkpoints enable cell cycle arrest, which provides time for repair and avoids further damage until the DNA damaging agent is cleared from the cell. Because DNA-damaging agents target DNA similarly in normal and cancer tissues, the effects of those clinically approved chemotherapeutic agents is likely to result from tumor-specific defects in DNA repair and DNA damage repair pathways.
Recent studies in breast and ovarian cancers suggest that targeting DNA repair may represent an efficient strategy for improving outcome of patients with metastatic cancers. Poly(ADP-ribose) polymerase 1 (PARP1) is a constitutively expressed nuclear enzyme important in base excision repair of single-stranded DNA breaks. In its absence, excessive single-strand breaks accumulate, leading to collapsed DNA replication forks and conversion of single-strand breaks to double-strand breaks (DSBs). BRCA1 and BRCA2 proteins repair double-strand DNA breaks by homologous recombination, and BRCA -defective cells are unable to repair these DSBs, resulting in tumor cell death. Inhibition of PARP1 in BRCA -deficient cells uses the concept of synthetic lethality, whereby a mutation in either of 2 genes individually has no effect on cell survival but combining the mutations leads to cell death. Inhibitors of PARP1 may offer an additional therapeutic option for BRCA carriers in breast and ovarian cancers Furthermore, expression of proteins involved in DNA repair pathways such as BRCA1/2, ERCC1, and MSH2 have been associated with response to platinum-based chemotherapy in lung cancer, bladder cancer, and triple-negative breast cancers.
The incidence of DNA repair defects and their clinical relevance in PCa remain to be clarified. BRCA1 and BRCA2 mutation carriers have an increased risk of developing prostate cancer. Germline BRCA mutations seem to predict aggressive disease, with carriers showing earlier progression through the prostate cancer clinical states model and increased prostate cancer–specific mortality than noncarriers. Although the incidence of BRCA2 mutation in prostate cancer is low, BRCA -associated prostate cancer may represent a genetically defined subset of the disease that may be more sensitive to platinum-based chemotherapy as observed in patients with BRCA -associated breast and ovarian cancers. Treatment of BRCA -associated prostate cancer with PARP inhibition would represent an efficient strategy of treatment tailored to BRCA2 status. Clinical trials assessing platinum-based agents such as carboplatin, cisplatin, or, more recently, satraplatin failed to show any benefit in patients with prostate cancer. In a recent retrospective study, response to taxane-based therapy defined by PSA nadir within 12 weeks of therapy was not associated with BRCA1/2 mutation status.
Oncogenic gene fusions recently found in PCa may also impair DNA repair ability of cancer cells. Oncogenic ETS gene rearrangements involving sequences of TMPRSS2 (an androgen-regulated gene) with ETS-family transcription factor genes (ERG, ETV1, ETV4, or ETV4) have been reported in around 40% of prostate cancers. Once an ETS gene fusion is formed through genomic rearrangement, the subsequent overexpression of an ETS gene fusion protein can contribute to cancer progression through various mechanisms. Overexpression of ERG leads to accelerated carcinogenesis in mouse prostates with deletion of the tumor suppressor PTEN. However, its clinical relevance remains to be elucidated because no strong association with clinical outcome has been shown. A recent study showed that androgen signaling promotes corecruitment of AR and topoisomerase II β (TOP2B) to sites of TMPRSS2-ERG genomic breakpoints, triggering recombinogenic TOP2B-mediated DSBs. Furthermore, androgen stimulation resulted in de novo production of TMPRSS2-ERG fusion transcripts in a process that required TOP2B and components of the DSB repair machinery. Another study showed that ERG interacts with the enzymes PARP1 and dependent protein kinase, catalytic subunit (DNAPKcs) involved in DNA repair pathways. Several PARP inhibitors such as olaparib or veliparib have been developed alone or in combination with conventional chemotherapy, especially in BRCA-mutated ovarian and breast cancers. In prostate cancer, pharmacologic inhibition of PARP1 preferentially sensitized ETS-overexpressing VCAP xenografts compared with ETS-negative xenografts (PC3, DU145, 22RV1), although the dose of olaparib (>10 μM) used in this study was probably beyond the recommended dose in the patients.
Clinical data
In the phase I clinical trial assessing olaparib in patients with BRCA1 or BRCA2 mutations, a patient with CRPC who was a BRCA2 mutation carrier had more than a 50% reduction in the PSA level and resolution of bone metastases, participating in the study for more than 58 weeks at the time of the cutoff date. A phase II assessing the combination of veliparib and the DNA damage agent, temozolomide, was conducted and is now closed to accrual (NCT01085422). Preliminary data showed that 2 patients out of 25 had a confirmed PSA response; 1 patient had a 37% decrease in PSA and the other patient had a 96% decrease in PSA and a 40% reduction in tumor size. Median PFS was 2.1 months (95% CI 1.8, 3.9).
Targeting cell proliferation
New Taxanes: Cabazitaxel
Preclinical data
Microtubules consist of tubulin heterodimers and radiate from the centrosome in the cytoplasm of interphase cells. They are involved in many cellular functions including cell motility, cell division, and intracellular transport. During the mitosis, microtubule networks ensure the correct attachment and segregation of chromosomes during cell division, making them attractive targets for anticancer drugs. Docetaxel is a taxane that binds to β-tubulin of microtubules during the G 2 -M phase of the cell cycle, leading to cell apoptosis in cycling cells. Recent studies have also shown additional effects of docetaxel on AR by preventing its translocation into the nucleus and thus inhibiting AR-induced signaling. Docetaxel-based chemotherapy is standard first-line chemotherapy in patients with metastatic CRPC (mCRPC). Two randomized phase III trials have shown an improved overall survival in chemonaive patients with CRPC treated with docetaxel alone or in combination with estramustine compared with mitoxantrone. However, almost 50% of patients do not respond to docetaxel.
Molecular mechanisms of resistance to docetaxel are multifactorial and involve decreased cellular drug accumulation caused by overexpression of membrane-bound efflux proteins (P-glycoprotein), expression of tubulin isotypes (overexpression of β-III tubulin) and defects in apoptosis. Some gene expression signatures have been associated with docetaxel resistance of prostate cancer cells, but no single driver pathway that responds to drugs has emerged to target and circumvent such resistance. New taxoid compounds have been developed as a low-affinity substrate for the P-glycoprotein efflux pump mechanism. Cabazitaxel (XRP6258, Jevtana) is a novel tubulin-binding taxane drug with antitumor activity in docetaxel-resistant cancers. Cabazitaxel promotes the assembly of tubulin and stabilizes microtubules against cold-induced depolymerization in vitro as potently as docetaxel. Cabazitaxel showed potent antitumor activity comparable with docetaxel in docetaxel-sensitive cell lines and exhibited more potent cytotoxic activity than docetaxel in cancer cell lines with acquired resistance to docetaxel caused by P-glycoprotein overexpression. Cabazitaxel resulted in antitumor activity on a broad array of tumor xenografts.
Clinical data
Phase 1 and 2 clinical studies reported that neutropenia is the primary dose-limiting toxicity, a recommended phase 2 dose between 20 and 25 mg/m 2 , and antitumor activity in solid tumors including docetaxel-refractory mCRPC, supporting further clinical investigations. Given the precedent efficacy of docetaxel in CRPC, along with the safety profile and observed responses in the phase I trial, an international phase III trial was conducted without phase II in patients with CRPC. The TROPIC trial was an international randomized phase 3 trial assessing cabazitaxel in men with mCRPC whose disease had progressed during or after treatment with a docetaxel-based regimen. Patients were randomly assigned to receive either cabazitaxel (25 mg mg/m 2 intravenously every 3 weeks) or mitoxantrone (12 mg/m 2 every 3 weeks). The primary end point was the overall survival (OS). This study showed that cabazitaxel improved median OS by 2.4 months (15.1 months vs 12.7 months) compared with the mitoxantrone group, with a hazard ratio (HR) reduction of 0.70 (95% confidence interval [CI] 0.59–0.83, P <.0001). Median progression-free survival (PFS) was also better in the cabazitaxel group (2.8 months, 95% CI 2.4–3.0) in the cabazitaxel group and 1.4 months (1.4–1.7 months) in the mitoxantrone group (HR 0.74, 0.64–0.86, P <.0001). The most common clinically significant grade 3 or higher adverse events were neutropenia (cabazitaxel 82% of patients vs mitoxantrone 58%) and diarrhea (6% vs <1%). A phase III trial is currently comparing cabazitaxel with docetaxel in chemonaive patients with mCRPC.
Epothilones
Preclinical data
Epothilones are, like taxanes, tubulin-binding agents but have different binding sites on the tubulin polymers. They induce apoptosis in cancer cells by disrupting the dynamic characteristics of microtubules. The epothilones include natural epothilone B (EPO906; patupilone) and several semisynthetic epothilone compounds such as BMS-247550 (ixabepilone) and sagopilone (ZK-EPO). Epothilones have cytotoxic effects against human cancer cells with high expression of P-glycoprotein and are also active in cancer cells resistant to taxanes because of tubulin mutations. Studies have shown that the precise binding sites and mode of action of epothilones differ from taxanes ; for example, ixabepilone can bind multiple β-tubulin isoforms, suppressing the dynamic instability of class III β-tubulin and class II β-tubulin microtubules, whereas taxanes weakly link class III β-tubulin. Epothilones also have low susceptibility to classic taxane resistance mechanisms, such as P-glycoprotein or multidrug resistance protein efflux, tubulin mutations, and alterations in tubulin isotypes.
Epothilone B (patupilone) has tumor activity in human prostate cancer DU-145 and PC-3 xenografts. Patupilone has antitumor activity against both paclitaxel-sensitive and paclitaxel-refractory cancers in vitro including in human cancer cell lines with P-glycoprotein overexpression and human cancer cell lines with tubulin mutations. Like epothilone B, ixabepilone has potent antitumor activity in mouse xenograft models bearing paclitaxel-sensitive or paclitaxel-refractory tumors. However, published preclinical data in prostate cancer xenografts are currently limited. Many studies have reported that ixabepilone has a low susceptibility to mechanisms of resistance to taxanes such as tubulin mutations and overexpression of the efflux pumps.
Sagopilone is a fully synthetic formulation of epothilone B. In vivo, sagopilone has shown activity in many cancers including prostate cancer, and cancer models with an multidrug-resistant phenotype and paclitaxel-resistant cells.
Clinical data
Phase I clinical trials indicate that epothilone and ixabepilone are well tolerated in patients who have cancer both as a single agent or in combination. However, neuropathy has led to frequent dose reductions in patients treated with ixabepilone in phase I clinical trials. The phase II clinical trials assessing ixabepilone suggest antitumor activity in men with mCRPC as single agent or in combination with estramustine. These agents have shown better activity in men with chemotherapy-naive mCRPC than in men previously treated with docetaxel. A phase II randomized study compared ixabepilone 35 mg/m 2 every 3 weeks with intravenous mitoxantrone 14 mg/m 2 every 3 weeks plus prednisone 5 mg twice daily in 82 patients with taxane-refractory CRPC. The efficacy end points were not different between the 2 arms. However, another phase II study showed that the combination of ixabepilone and mitoxantrone is both feasible and active in CRPC.
A phase II trial of patupilone (8 mg/m 2 ) was safe, had antitumor activity, and was associated with symptomatic improvement in patients previously treated with docetaxel. A prostate-specific antigen (PSA) decline of greater than or equal to 50% occurred in 47% of patients, whereas a partial measurable disease response occurred in 24% of assessable patients. A patient-reported pain response was also observed in 59% of assessable patients. In a recent phase II study, the activity of sagopilone 16 mg/m 2 given intravenously over 3 hours every 21 days was investigated in chemotherapy-naive patients with mCRPC in combination with oral prednisone. Of the 24 patients evaluable for response, 5 (21%) achieved a PSA response (reduction ≥50%) and 14 (58%) had a 30% PSA reduction within 3 months of enrollment. Among the 12 patients with measurable disease, there was 1 confirmed complete response (CR) and 1 confirmed partial response (PR), and 4 further patients achieved unconfirmed PRs.
Third-generation taxanes such as TPI 287 and agents targeting the mitotic process (ispinesib targets kinesin spindle protein, and danusertib targets the serine-threonine aurora kinase) are currently investigated in phase II clinical trials in men with mCRPC previously treated with docetaxel.
DNA Repair Inhibition
Preclinical data
DNA repair is essential because DNA is susceptible to spontaneous damage. Cellular DNA is susceptible to carcinogens, and the target of a broad range of anticancer agents. Several cancer susceptibility genes therefore encode for DNA repair and DNA damage response factors. DNA repair is coupled with DNA damage responses that are commonly referred to as checkpoint responses. Those checkpoints enable cell cycle arrest, which provides time for repair and avoids further damage until the DNA damaging agent is cleared from the cell. Because DNA-damaging agents target DNA similarly in normal and cancer tissues, the effects of those clinically approved chemotherapeutic agents is likely to result from tumor-specific defects in DNA repair and DNA damage repair pathways.
Recent studies in breast and ovarian cancers suggest that targeting DNA repair may represent an efficient strategy for improving outcome of patients with metastatic cancers. Poly(ADP-ribose) polymerase 1 (PARP1) is a constitutively expressed nuclear enzyme important in base excision repair of single-stranded DNA breaks. In its absence, excessive single-strand breaks accumulate, leading to collapsed DNA replication forks and conversion of single-strand breaks to double-strand breaks (DSBs). BRCA1 and BRCA2 proteins repair double-strand DNA breaks by homologous recombination, and BRCA -defective cells are unable to repair these DSBs, resulting in tumor cell death. Inhibition of PARP1 in BRCA -deficient cells uses the concept of synthetic lethality, whereby a mutation in either of 2 genes individually has no effect on cell survival but combining the mutations leads to cell death. Inhibitors of PARP1 may offer an additional therapeutic option for BRCA carriers in breast and ovarian cancers Furthermore, expression of proteins involved in DNA repair pathways such as BRCA1/2, ERCC1, and MSH2 have been associated with response to platinum-based chemotherapy in lung cancer, bladder cancer, and triple-negative breast cancers.
The incidence of DNA repair defects and their clinical relevance in PCa remain to be clarified. BRCA1 and BRCA2 mutation carriers have an increased risk of developing prostate cancer. Germline BRCA mutations seem to predict aggressive disease, with carriers showing earlier progression through the prostate cancer clinical states model and increased prostate cancer–specific mortality than noncarriers. Although the incidence of BRCA2 mutation in prostate cancer is low, BRCA -associated prostate cancer may represent a genetically defined subset of the disease that may be more sensitive to platinum-based chemotherapy as observed in patients with BRCA -associated breast and ovarian cancers. Treatment of BRCA -associated prostate cancer with PARP inhibition would represent an efficient strategy of treatment tailored to BRCA2 status. Clinical trials assessing platinum-based agents such as carboplatin, cisplatin, or, more recently, satraplatin failed to show any benefit in patients with prostate cancer. In a recent retrospective study, response to taxane-based therapy defined by PSA nadir within 12 weeks of therapy was not associated with BRCA1/2 mutation status.
Oncogenic gene fusions recently found in PCa may also impair DNA repair ability of cancer cells. Oncogenic ETS gene rearrangements involving sequences of TMPRSS2 (an androgen-regulated gene) with ETS-family transcription factor genes (ERG, ETV1, ETV4, or ETV4) have been reported in around 40% of prostate cancers. Once an ETS gene fusion is formed through genomic rearrangement, the subsequent overexpression of an ETS gene fusion protein can contribute to cancer progression through various mechanisms. Overexpression of ERG leads to accelerated carcinogenesis in mouse prostates with deletion of the tumor suppressor PTEN. However, its clinical relevance remains to be elucidated because no strong association with clinical outcome has been shown. A recent study showed that androgen signaling promotes corecruitment of AR and topoisomerase II β (TOP2B) to sites of TMPRSS2-ERG genomic breakpoints, triggering recombinogenic TOP2B-mediated DSBs. Furthermore, androgen stimulation resulted in de novo production of TMPRSS2-ERG fusion transcripts in a process that required TOP2B and components of the DSB repair machinery. Another study showed that ERG interacts with the enzymes PARP1 and dependent protein kinase, catalytic subunit (DNAPKcs) involved in DNA repair pathways. Several PARP inhibitors such as olaparib or veliparib have been developed alone or in combination with conventional chemotherapy, especially in BRCA-mutated ovarian and breast cancers. In prostate cancer, pharmacologic inhibition of PARP1 preferentially sensitized ETS-overexpressing VCAP xenografts compared with ETS-negative xenografts (PC3, DU145, 22RV1), although the dose of olaparib (>10 μM) used in this study was probably beyond the recommended dose in the patients.
Clinical data
In the phase I clinical trial assessing olaparib in patients with BRCA1 or BRCA2 mutations, a patient with CRPC who was a BRCA2 mutation carrier had more than a 50% reduction in the PSA level and resolution of bone metastases, participating in the study for more than 58 weeks at the time of the cutoff date. A phase II assessing the combination of veliparib and the DNA damage agent, temozolomide, was conducted and is now closed to accrual (NCT01085422). Preliminary data showed that 2 patients out of 25 had a confirmed PSA response; 1 patient had a 37% decrease in PSA and the other patient had a 96% decrease in PSA and a 40% reduction in tumor size. Median PFS was 2.1 months (95% CI 1.8, 3.9).
Targeting survival pathways
Defects in the ability to appropriately regulate apoptotic processes are one of the fundamental properties underlying cancer. Bcl-2 was the first identified member of a family of apoptotic regulators sharing at least 1 Bcl-2 homology domain. Bcl-2 family members include antiapoptotic proteins (eg, Bcl-2, Bcl-X L , and Mcl-1), multidomain proapoptotic proteins (eg, Bax and Bak), and BH3-only proapoptotic proteins (eg, Bim, Bid, Noxa, and Puma). Interactions between, and relative ratios of, proapoptotic and antiapoptotic Bcl-2 family members are key determinants of cellular sensitivity to multiple cell death triggers, including many standard chemotherapeutic agents and ionizing radiation (IR). Bcl-2, Bcl-X L , and Mcl-1 gene amplification have been shown to be associated with castration resistance. Targeting such antiapoptotic proteins resulted in improvement of chemotherapy efficacy in PCa cell lines.
BCL-2
Preclinical data
Bcl-2 antisense oligodeoxynucleotides (ASO) (G3139, oblimersen sodium, Genasense) have been shown to be effective in reducing Bcl-2 expression in several cell lines including prostate cancer cells. Bcl-2 ASO enhances chemotherapy and radiation-induced cytotoxicity in prostate cancer cell lines. Recently, BH3 mimetics such as ABT-737 and AT-101 (R-(−)-gossypol) have been designed to inhibit heterodimerization of Bcl-2 or Bcl-X L to proapoptotic BH3 family members (Bax, Bak), resulting in caspase-dependent apoptosis activation.
The BH3 mimetic ABT-737 binds with high affinity to the hydrophobic cleft and BH3 receptor region of Bcl-2, Bcl-X L , and Bcl-w, but not to the less homologous Bcl-2–related protein Mcl-1. This ABT-737/Bcl-2 interaction antagonizes the interaction of Bcl-2 with the BH3 domain of proapoptotic proteins, neutralizing Bcl-2. Although ABT-737 shows single-agent activity promoting apoptosis in human small cell lung cancer and lymphoma cell lines in vitro and in tumor xenografts, apoptosis is not triggered in most human cancer cell lines. Expression of Mcl-1, which is not targeted by ABT-737, may explain the resistance in prostate and other cancer cell lines to apoptosis. However, combining ABT-737 with agents that target Mcl-1 sensitized prostate cancer cell lines with an apoptotic block to cell death in vitro.
Clinical data
The phase I clinical trial combining oblimersen and docetaxel did not show any severe toxicities at recommended dose and showed evidence of Bcl-2 protein inhibition in tumor tissue, and encouraging antitumor activity in patients with CRPC. However, a randomized, phase II study failed to confirm preliminary data, mostly because of its short tissue half-life and interruptions with its continuous infusion, leading to insufficient target inhibition.
A phase I/II trial combining docetaxel day 1 every 21 days with AT-101 40 mg twice daily on days 1 to 3 in chemonaive men with mCRPC showed encouraging efficacy, with two-thirds of patients achieving a biochemical response defined as decline greater than or equal to 50% and some of them (45%) having a measurable PR. However, these preliminary results were not confirmed in a phase 2 trial that recently reported that AT-101 failed to improve OS when combined with docetaxel-based chemotherapy (NCT00286793). The more selective compound ABT-737 and its oral-derived enantiomer ABT-263 are currently being assessed in many phase I and phase II clinical trials based on preclinical data. A phase I trial is investigating the combination of docetaxel and ABT-263 in solid tumors including PCa (NCT00888108). However, the frequent pitfalls of such studies are the unselected population of patients and an empiric treatment sequence when the Bcl-2/Bcl-X L inhibitors are given in combination.
Clusterin
Preclinical data
Stress-induced prosurvival gene and cytoprotective chaperone networks are mechanisms involved in resistance to androgen deprivation therapy and chemotherapy in prostate and other cancers. Secretory clusterin (sCLU) is a multifunctional, stress-induced, adenosine triphosphate (ATP)–independent molecular chaperone involved in many biologic processes ranging from mammary and prostate gland involution to amyloidosis and neurodegenerative disease, as well as cancer progression and treatment resistance. sCLU functions to protect cells from many varied therapeutic stressors that induce apoptosis, including androgen or estrogen withdrawal, radiation, cytotoxic chemotherapy, and biologic agents. sCLUs interact with stressed cell surface proteins to inhibit proapoptotic signal transduction ( Fig. 2 ). sCLU inhibits endoplasmic reticulum (ER) stress, retrotranslocating from the ER to the cytosol to inhibit aggregation of intracellular proteins and prevent apoptosis. sCLU inhibits mitochondrial apoptosis by increasing Akt phosphorylation levels and NF-κB nuclear transactivation.









