The treatment of metastatic castration-resistant prostate cancer has evolved since the approval of docetaxel-based therapy. Since docetaxel approval, three new agents have gained approval for this indication: sipuleucel-T, cabazitaxel, and abiraterone. Recent Phase III trials have also demonstrated survival benefits for MDV-3100 and radium-223 though regulatory approval ispending. Practicing physicians face the challenge of determining the optimal sequencing of these new agents. This dilemma is particularly relevant to the post-docetaxel setting, in which the indication for several of these agents overlaps. This article details the efficacy and safety of these agents to provide a framework for their clinical use.
- •
Multiple new agents are available for metastatic castrate resistant prostate cancer.
- •
Abiraterone and cabazitaxel are FDA approved in the post-docetaxel space.
- •
Sipuleucel-T is FDA approved for patients with metastatic CRPC that have minimal or no symptoms.
- •
MDV-3100 and radium-223 are two additional agents that have also reported positive phase 3 trials for metastatic CRPC.
- •
No comparative trials have been conducted between any of these newer agents, leaving clinicians with many questions.
Introduction
In 1941, Huggins and colleagues noted the dramatic effects of surgical castration in the treatment of metastatic prostate cancer. This landmark development revolutionized the therapy of the disease, and over time, pharmacologic methods of castration were developed as an alternative to surgical methods. Huggins recognized early on that “despite regressions of great magnitude, it is obvious that there are many failures of endocrine therapy to control the disease.” Though this disease state has undergone several nomenclature changes (including androgen-independent prostate cancer and hormone refractory prostate cancer), today we refer to this disease state as castration-resistant prostate cancer (CRPC), which may or not be metastatic. The onset of the metastatic CRPC (mCRPC) occurs at a median of about 9 years after androgen-deprivation therapy (ADT) for patients initially treated for nonmetastatic disease or 1 to 3 years after ADT for patients initially treated for metastatic disease.
For decades, clinical trials failed to show a definitive advantage with novel therapies for mCRPC. Two pivotal trials examining docetaxel-based regimens were the first to demonstrate an overall survival benefit in patients with mCRPC. In the TAX 327 trial, patients were randomized to receive either mitoxantrone-prednisone or docetaxel-prednisone in one of two schedules. Ultimately, docetaxel at a dose of 75 mg/m intravenously (IV) every 3 weeks with prednisone daily led to a survival advantage over standard mitoxantrone-prednisone therapy (18.9 months vs 16.5 months, P = .009). In Southwest Oncology Group (SWOG) trial, 9916 patients were randomized to receive either docetaxel-estramusine or mitoxantrone-prednisone. Again, a survival advantage was noted with docetaxel-based therapy (17.5 months vs 15.6 months, P = .02). The cumulative data from these studies led to the approval of docetaxel-prednisone–based therapy for mCRPC on May 19, 2004.
In the ensuing years, several different approaches were taken to build on the success of docetaxel in patients with mCRPC. One approach was to explore combinations of novel therapies with docetaxel. Unfortunately, to date, these studies have proven somewhat disappointing. A second approach to the patient with mCRPC has been to explore the efficacy of novel therapies either before or after docetaxel therapy. These efforts have thus far proven to be more fruitful than explorations of combination therapy. To date, the bulk of progress has been made in the postdocetaxel space, in which agents such as cabazitaxel, abiraterone, MDV3100, and radium-223 have demonstrated statistically significant benefits in overall survival. Thus far, cabazitaxel-prednisone and abiraterone-prednisone are FDA approved. Although some agents (ie, abiraterone, radium-223) may ultimately straddle predocetaxel and postdocetaxel spaces, this article focuses on the current postdocetaxel strategies. The clinical data related to each agent is reviewed in detail to provide the physician with a framework with which to approach the docetaxel-refractory patient.
Cabazitaxel
Structurally, docetaxel and the novel taxane cabazitaxel are similar, with two hydroxyl side chains (docetaxel) substituted for two methoxy groups (cabazitaxel) ( Fig. 1 ). Both exert their preclinical activity through inhibition of microtubule disassembly, akin to other taxanes. However, early in its development, the preclinical activity of cabazitaxel was noted to be distinct from docetaxel. In a variety of cell lines (including P388 [lymphoblastic leukemia], HL60 [promyelocytic leukemia], and Calc18 [breast adenocarcinoma] models), cabazitaxel was noted to inhibit growth at relatively low concentrations, with mean inhibitory concentration in the range of 3 to 29 nM. The antitumor activity of cabazitaxel was maintained in cell lines that were resistant to other standard taxanes as well. In murine xenograft models of prostate cancer (including the hormone-resistant DU145 cell line), near complete tumor regressions were observed.
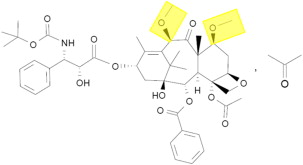
The encouraging preclinical data for cabazitaxel led to initiation of a phase I clinical trial. In this study, 25 patients with advanced solid tumors were treated with doses of cabazitaxel ranging from 10 to 25 mg/m 2 . Eight patients with advanced prostate cancer were enrolled on the study, representing the largest subgroup based on tumor type. Pharmacokinetic data from this study indicated a triphasic model of elimination (t 1/2 = 2.5 minutes, 1.3 hours, and 77.3 hours for the three phases, respectively). Notably, the recommended phase II dose emerging from the study was 20 mg/m 2 , given dose-limiting toxicities of febrile neutropenia and grade 4 neutropenia occurring at a dose of 25 mg/m 2 . Two patients attained a partial response in this phase I effort—both patients with advanced prostate cancer who had received prior mitoxantrone and docetaxel.
A separate phase I study examined doses of cabazitaxel ranging from 10 to 30 mg/m 2 IV every 3 weeks; the recommended phase II dose in this effort was 25 mg/m 2 . Akin to the previously noted phase I study, the most frequent toxicities were neutropenia, febrile neutropenia, diarrhea, and infection. This study helped establish the extent of ex vivo plasma protein binding (approximately 92%); cabazitaxel showed high binding to both lipoproteins and albumin. The intrapatient variability of the area under the curve between 0 to 48 hours (AUC 0–48 ) was also ascertained in this study and was estimated to be approximately 27%.
A phase II exploration of cabazitaxel in heavily pretreated breast cancer used a phase II dose of 20 mg/m 2 as a starting dose but allowed escalation to 25 mg/m 2 provided tolerance of the initial dose level. Although originally designed as a three-arm, randomized phase II study evaluating both cabazitaxel and a distinct novel taxane (larotaxel), the trial design was modified owing to poor accrual to include just one arm. Patients may have received prior taxane in either the adjuvant or metastatic setting. With a total of 67 patients, the overall response rate was 14%, and the median duration of response was 7.6 months (range, 2.6–18.7 months). Although two complete responses were observed, several concerning safety signals were seen in this study, with two deaths due to nonhematologic toxicity noted within 30 days of study treatment.
A rather unique element of the clinical development of cabazitaxel is its evolution from phase I to phase III assessment in prostate cancer—there was no phase II evaluation of the drug outside of the setting of breast cancer. The phase III TROPIC study built on the preclinical efficacy of cabazitaxel seen in preclinical models of hormone-resistant prostate cancer and the responses seen in the phase I assessment. In this study, 775 patients with docetaxel refractory disease were randomized to receive either cabazitaxel at 25 mg/m 2 IV every 3weeks with prednisone or mitoxantrone with prednisone. The definition of docetaxel-refractory disease used employed in TROPIC included either (1) response evaluation criteria in solid tumors–based progression, or (2) two consecutive prostate-specific antigen (PSA) rises at least 1 week apart. Patients were initially permitted to enroll with any prior docetaxel exposure, but the study was later modified to include patients who had at least a cumulative docetaxel dose of 225 mg/m 2 .
The study met its primary end point, demonstrating a significant improvement in median overall survival from 12.7 months with mitoxantrone-prednisone to 15.1 months with cabazitaxel-prednisone ( P <.001). Furthermore, progression-free survival was improved with cabazitaxel-based therapy (2.8 months vs 1.4 months, P <.0001). Pain relief, as assessed by the McGill-Melzack pain questionnaire, was not distinct between arms. The positive survival advantage led to the FDA approval of cabazitaxel on June 17, 2010. Interestingly, post hoc analyses show that this survival advantage actually extends to the overall survival time from first docetaxel usage. Specifically, median overall survival was 29 months from the time of first docetaxel use in patients receiving subsequent cabazitaxel therapy compared with 25 months in patients receiving subsequent mitoxantrone therapy. Furthermore, a survival benefit was seen in subgroups that discontinued use of docetaxel therapy for progression and in subgroups that discontinued use of docetaxel therapy for other reasons (eg, adverse events, intolerance). The findings in patients with progression while on docetaxel underscore the distinction between docetaxel and cabazitaxel.
As in earlier experiences with cabazitaxel, the most common adverse event noted in the phase III study was neutropenia, with grade greater than or equal to 3 neutropenia occurring in 82% of patients and 8% of patients developing febrile neutropenia. One factor that may account for the high frequency of neutropenia and related events seen in TROPIC was the prohibition of prophylactic growth factor use. With the first cycle of therapy with cabazitaxel, patients were not allowed to use these agents, although they could be implemented subsequently. Recommendations accompanying the FDA approval of cabazitaxel recommend use of growth factor support in at-risk groups, including (1) older patients (age >65), (2) recipients of extensive prior radiation, (3) patients with poor nutritional status, (4) patients with prior documented episodes of febrile neutropenia, (5) patients with poor performance status, and (6) patients with other serious medical comorbidities. It is foreseeable that these criteria encompass most patients in a typical prostate cancer clinic. A retrospective subset analysis of patients who received prophylactic growth factor support from cycle 2 of cabazitaxel therapy onwards did suggest a dramatic decline in grade greater than 3 neutropenia (23.7% vs 57.7%; P <.0001). These data support the more aggressive use of growth factors in many patients receiving cabazitaxel therapy today in the clinic.
Other notable toxicities associated with cabazitaxel include diarrhea—several deaths secondary to treatment-related diarrhea were seen in the TROPIC study. Recommendations accompanying the publication of the TROPIC data suggest vigilant monitoring for dehydration, with prompt administration of antidiarrheals and fluids if this toxicity is incurred. Other frequent nonhematologic toxicities include fatigue and asthenias. With the caveats of cross-trial comparisons, several toxicities (ie, neuropathy and alopecia) seem less prevalent with cabazitaxel therapy.
Cabazitaxel
Structurally, docetaxel and the novel taxane cabazitaxel are similar, with two hydroxyl side chains (docetaxel) substituted for two methoxy groups (cabazitaxel) ( Fig. 1 ). Both exert their preclinical activity through inhibition of microtubule disassembly, akin to other taxanes. However, early in its development, the preclinical activity of cabazitaxel was noted to be distinct from docetaxel. In a variety of cell lines (including P388 [lymphoblastic leukemia], HL60 [promyelocytic leukemia], and Calc18 [breast adenocarcinoma] models), cabazitaxel was noted to inhibit growth at relatively low concentrations, with mean inhibitory concentration in the range of 3 to 29 nM. The antitumor activity of cabazitaxel was maintained in cell lines that were resistant to other standard taxanes as well. In murine xenograft models of prostate cancer (including the hormone-resistant DU145 cell line), near complete tumor regressions were observed.
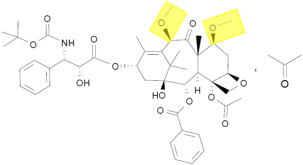
The encouraging preclinical data for cabazitaxel led to initiation of a phase I clinical trial. In this study, 25 patients with advanced solid tumors were treated with doses of cabazitaxel ranging from 10 to 25 mg/m 2 . Eight patients with advanced prostate cancer were enrolled on the study, representing the largest subgroup based on tumor type. Pharmacokinetic data from this study indicated a triphasic model of elimination (t 1/2 = 2.5 minutes, 1.3 hours, and 77.3 hours for the three phases, respectively). Notably, the recommended phase II dose emerging from the study was 20 mg/m 2 , given dose-limiting toxicities of febrile neutropenia and grade 4 neutropenia occurring at a dose of 25 mg/m 2 . Two patients attained a partial response in this phase I effort—both patients with advanced prostate cancer who had received prior mitoxantrone and docetaxel.
A separate phase I study examined doses of cabazitaxel ranging from 10 to 30 mg/m 2 IV every 3 weeks; the recommended phase II dose in this effort was 25 mg/m 2 . Akin to the previously noted phase I study, the most frequent toxicities were neutropenia, febrile neutropenia, diarrhea, and infection. This study helped establish the extent of ex vivo plasma protein binding (approximately 92%); cabazitaxel showed high binding to both lipoproteins and albumin. The intrapatient variability of the area under the curve between 0 to 48 hours (AUC 0–48 ) was also ascertained in this study and was estimated to be approximately 27%.
A phase II exploration of cabazitaxel in heavily pretreated breast cancer used a phase II dose of 20 mg/m 2 as a starting dose but allowed escalation to 25 mg/m 2 provided tolerance of the initial dose level. Although originally designed as a three-arm, randomized phase II study evaluating both cabazitaxel and a distinct novel taxane (larotaxel), the trial design was modified owing to poor accrual to include just one arm. Patients may have received prior taxane in either the adjuvant or metastatic setting. With a total of 67 patients, the overall response rate was 14%, and the median duration of response was 7.6 months (range, 2.6–18.7 months). Although two complete responses were observed, several concerning safety signals were seen in this study, with two deaths due to nonhematologic toxicity noted within 30 days of study treatment.
A rather unique element of the clinical development of cabazitaxel is its evolution from phase I to phase III assessment in prostate cancer—there was no phase II evaluation of the drug outside of the setting of breast cancer. The phase III TROPIC study built on the preclinical efficacy of cabazitaxel seen in preclinical models of hormone-resistant prostate cancer and the responses seen in the phase I assessment. In this study, 775 patients with docetaxel refractory disease were randomized to receive either cabazitaxel at 25 mg/m 2 IV every 3weeks with prednisone or mitoxantrone with prednisone. The definition of docetaxel-refractory disease used employed in TROPIC included either (1) response evaluation criteria in solid tumors–based progression, or (2) two consecutive prostate-specific antigen (PSA) rises at least 1 week apart. Patients were initially permitted to enroll with any prior docetaxel exposure, but the study was later modified to include patients who had at least a cumulative docetaxel dose of 225 mg/m 2 .
The study met its primary end point, demonstrating a significant improvement in median overall survival from 12.7 months with mitoxantrone-prednisone to 15.1 months with cabazitaxel-prednisone ( P <.001). Furthermore, progression-free survival was improved with cabazitaxel-based therapy (2.8 months vs 1.4 months, P <.0001). Pain relief, as assessed by the McGill-Melzack pain questionnaire, was not distinct between arms. The positive survival advantage led to the FDA approval of cabazitaxel on June 17, 2010. Interestingly, post hoc analyses show that this survival advantage actually extends to the overall survival time from first docetaxel usage. Specifically, median overall survival was 29 months from the time of first docetaxel use in patients receiving subsequent cabazitaxel therapy compared with 25 months in patients receiving subsequent mitoxantrone therapy. Furthermore, a survival benefit was seen in subgroups that discontinued use of docetaxel therapy for progression and in subgroups that discontinued use of docetaxel therapy for other reasons (eg, adverse events, intolerance). The findings in patients with progression while on docetaxel underscore the distinction between docetaxel and cabazitaxel.
As in earlier experiences with cabazitaxel, the most common adverse event noted in the phase III study was neutropenia, with grade greater than or equal to 3 neutropenia occurring in 82% of patients and 8% of patients developing febrile neutropenia. One factor that may account for the high frequency of neutropenia and related events seen in TROPIC was the prohibition of prophylactic growth factor use. With the first cycle of therapy with cabazitaxel, patients were not allowed to use these agents, although they could be implemented subsequently. Recommendations accompanying the FDA approval of cabazitaxel recommend use of growth factor support in at-risk groups, including (1) older patients (age >65), (2) recipients of extensive prior radiation, (3) patients with poor nutritional status, (4) patients with prior documented episodes of febrile neutropenia, (5) patients with poor performance status, and (6) patients with other serious medical comorbidities. It is foreseeable that these criteria encompass most patients in a typical prostate cancer clinic. A retrospective subset analysis of patients who received prophylactic growth factor support from cycle 2 of cabazitaxel therapy onwards did suggest a dramatic decline in grade greater than 3 neutropenia (23.7% vs 57.7%; P <.0001). These data support the more aggressive use of growth factors in many patients receiving cabazitaxel therapy today in the clinic.
Other notable toxicities associated with cabazitaxel include diarrhea—several deaths secondary to treatment-related diarrhea were seen in the TROPIC study. Recommendations accompanying the publication of the TROPIC data suggest vigilant monitoring for dehydration, with prompt administration of antidiarrheals and fluids if this toxicity is incurred. Other frequent nonhematologic toxicities include fatigue and asthenias. With the caveats of cross-trial comparisons, several toxicities (ie, neuropathy and alopecia) seem less prevalent with cabazitaxel therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






