Chapter 87 Systemic chemotherapy for hepatic colorectal cancer
Impact on surgical management
Overview
The liver is the most common site of metastases from colorectal cancer (CRC). Approximately half of all patients with CRC will develop liver metastases at some point during their disease (Ekberg et al, 1987; Steele & Ravikumar, 1989), and half of these will be seen with synchronous liver metastases at the time of diagnosis (Bengmark & Hafstrom, 1969). Resection remains the best treatment for patients with resectable colorectal liver metastases (CLM; see Chapter 81A). Early studies reported 5-year survival rates of 25% to 48% following complete resection of the liver metastases (Adson et al, 1984; Fong et al, 1997; Sugihara et al, 1993) in an era when the only active chemotherapeutic agent used in the United States was the antimetabolite 5-fluorouracil (5-FU). With the single-agent regimen of 5-FU, or 5-FU plus leucovorin (LV), tumor response was seen in only 10% to 20% of those treated, which provided an overall survival of approximately a year in patients who were not candidates for an operation (Meta-Analysis Group in Cancer [MAGIC], 1998; Thirion et al, 2004). Substantial improvements in both response and survival have been reported with the use of the two “modern” cytotoxic agents, irinotecan and oxaliplatin.
Newer chemotherapeutic regimens that combine continuously infused 5-FU and LV with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) yield overall response rates over 50% and provide a doubling of survival time in patients with unresectable disease (de Gramont et al, 2000; Tournigand et al, 2004). The integration of molecularly targeted agents, such as bevacizumab (monoclonal antibody to vascular endothelial growth factor) and cetuximab (antiepidermal growth factor receptor), into treatment strategies has further increased response rates to an impressive 70% (Folprecht et al, 2010). By combining surgery with these newer chemotherapeutic agents and regimens, 5-year survival rates approaching 60% have been reported following hepatic resection of CLM (Abdalla et al, 2004; Choti et al, 2002; Fernandez et al, 2004).
Chemotherapeutic Agents
Fluoropyrimidines
The fluoropyrimidine 5-FU has remained the backbone of systemic chemotherapy for metastatic CRC for decades. This drug inhibits thymidylate synthase, thereby lowering production of the pyrimidine thymidine for DNA synthesis. It has been used synergistically with leucovorin (folate) either in a daily bolus administration in repeated cycles of 5 days (Mayo Clinic regimen) or on a biweekly infusional schedule (de Gramont regimen). By meta-analysis, continuous infusional administration of 5-FU/LV provides an improved response rate (24% vs. 14% with bolus; P = .002) and decreased grade 3 to 4 toxicity (4% vs. 31% with bolus; P < .001) compared with bolus therapy (MAGIC, 1998; de Gramont et al, 1997). Capecitabine is the orally administered prodrug of 5-FU, which has lower toxicity and provides similar survival rates compared with an intravenous 5-FU/LV regimen (Hoff et al, 2001).
Irinotecan
Irinotecan (CPT-11) is a topoisomerase I inhibitor, which prevents DNA unwinding and results in failure of DNA replication, DNA strand breaks, and cell death. In a randomized trial that evaluated irinotecan in patients with unresectable metastatic CRC, survival after irinotecan alone was equivalent to that of patients treated with standard bolus 5-FU/LV, but the combination of irinotecan and 5-FU/LV improved overall survival by 2.2 months over 5-FU/LV alone (14.8 vs. 12.6 months; P = .04) (Saltz et al, 2000). Subsequently, the combination of irinotecan with 5-FU/LV administered in continuous infusion (FOLFIRI) was associated with further improvement in survival (17.4 vs. 14.1 months; P = .03) (Douillard et al, 2000).
Oxaliplatin
Oxaliplatin is a platinum compound that alkylates DNA strands, thereby inhibiting DNA transcription and replication, resulting in cell death. Although already in use in Europe, oxaliplatin received U.S. Food and Drug Administration (FDA) approval in 2002. Oxaliplatin is commonly used in combination with continuous infusional 5-FU/LV (FOLFOX). Different drug doses and timed regimens—FOLFOX4, FOLFOX6, and FOLFOX7—have been evaluated, but no data support the superiority of any one over another in terms of patient survival. In a national effort, the intergroup N9741 trial (Goldberg et al, 2004) showed that FOLFOX treatment was associated with a 4.5-month improvement in median overall survival compared with continuous infusion of 5-FU/LV alone (19.5 vs. 15 months; P = .001). As no trial has found a difference in efficacy between FOLFOX and FOLFIRI regimens, both remain first-line treatment options for systemic chemotherapy treatment of unresectable metastatic CRC. FOLFOX is used primarily over FOLFIRI because of potentially debilitating diarrhea associated with the latter.
Molecular Targeted Agents
The monoclonal antibodies bevacizumab and cetuximab have emerged as useful adjuncts to systemic chemotherapy for metastatic CRC. Bevacizumab targets and effectively neutralizes circulating vascular endothelial growth factor (VEGF), and several mechanisms of action have been proposed. VEGF is one of the key growth factors required for development and stabilization of new blood vessels that supply tumor growth. Bevacizumab decreases microvascular density and integrity and reduces interstitial tumor pressure in vivo, potentially increasing local delivery of other concurrently administered cytotoxic drugs (Jain, 2001; Willett et al, 2004).
In 2004 Hurwitz and colleagues demonstrated a benefit of adding bevacizumab to systemic chemotherapy for metastatic CRC. In a randomized controlled double-blinded trial, they compared bolus 5-FU/LV plus irinotecan in conjunction with either bevacizumab (n = 402) or placebo (n = 411). Patients in the bevacizumab group had a near 5-month improvement in median survival compared with the placebo group (20.3 vs. 15.6 months; P < .001), and they had an increase in median progression-free survival (10.6 vs. 6.5 months; P = < .001). Although generally well tolerated, the toxicity seen in the bevacizumab group was that of increased gastrointestinal perforation (1.5% of patients) and major thrombotic events (2.5%). In addition, treatment with bevacizumab prior to surgery may be associated with increased wound infections and dehiscences, attributable to inhibition of neovascularization of the healing wound (Scappaticci et al, 2005); however, following hepatic resection for CLM, this phenomenon was not observed in a prospective Phase II trial, in which bevacizumab had been discontinued for 5 weeks (Gruenberger et al, 2008). The current recommendation is still to wait at least 28 days from cessation of bevacizumab to proceed with surgery, but many surgeons prefer to wait 6 weeks.
Cetuximab and the newer drug panitumumab are two recombinant chimeric monoclonal antibodies, which bind to the epidermal growth factor receptor (EGFR). Cetuximab is sometimes used as first-line therapy of unresectable metastatic colon cancer. By competitively blocking the transmembrane tyrosine kinase EGFR, these agents inhibit cell growth, induce apoptosis, and decrease matrix metalloproteinase and VEGF production; however, the effectiveness of both of these drugs seems to be significantly dependent on the wild-type phenotype of KRAS, as this protein is part of the downstream signal-transduction pathway of EGFR. Results from the CRYSTAL study showed that for patients with wild-type KRAS, the addition of cetuximab to FOLFIRI as first-line treatment for CLM increased the response rate to 59% compared with 43% using FOLFIRI alone (P = .003). A slight increase in progression-free survival was found (9.9 vs. 8.7 months; P = .017), a benefit not seen among patients with KRAS mutations (Van Cutsem et al, 2008). The OPUS study was constructed similarly and assessed the addition of cetuximab to FOLFOX, and it also showed benefits for the KRAS wild-type subgroup (61% vs. 37%; P = .011) but not for patients with KRAS mutations (Bokemeyer et al, 2009). Based on these results, the American Society of Clinical Oncology published a consensus statement that patients who have tumors with KRAS mutations in codon 12 or 13 were unaffected by EGFR inhibition, and they recommended that anti-EGFR drugs not be used as part of the treatment regimen for this patient population (Allegra et al, 2009).
The colorectal liver metastases (CELIM) study randomized patients with unresectable colorectal liver metastases to receive FOLFOX plus cetuximab or FOLFIRI plus cetuximab in a nonblinded fashion. Response rates for the 53 patients in each arm were similar (68% and 57%; P = not significant [NS]). When analyzed for KRAS mutation, an impressive overall response rate of 70% was found for the entire group (Folprecht et al, 2010). Following a median of eight cycles, complete resection was achieved in 41 patients (34%), suggesting that this regimen was useful for converting unresectable cases to resectable ones. In a secondary analysis, 68 patients were included in a retrospective reevaluation by seven experienced hepatobiliary surgeons. Twenty-two patients (32%) were considered to be resectable based on initial staging imaging studies, which increased to 41 patients (60%) based on restaging studies after chemotherapy (P = .001).
Conversion Therapy
The first retrospective reports from France (Adam et al, 2001; Bismuth et al, 1996; Giacchetti et al, 1999) were updated in 2004 with a series of 1439 patients who underwent resection of colorectal liver metastases between 1988 and 1999. Among these, 1104 patients were described who were initially considered to have unresectable disease based on large tumor size, poor tumor location, multinodularity, or presence of extrahepatic disease. After receiving an average of 10 cycles of systemic chemotherapy (5-FU and chronomodulated oxaliplatin), 138 patients (12.5%) demonstrated enough response to become anatomically resectable, but within the median follow-up time of 49 months, 111 (80%) recurred. Recurrence was limited to the liver in 29%. Five-year overall and disease-free survival were 33% and 22%, both of which were lower than for those patients considered resectable prior to chemotherapy (48% and 30%, respectively; P = .01). The authors identified four preoperative factors independently associated with shorter survival: a rectal primary, three or more metastases, maximum tumor size greater than 10 cm, and a carbohydrate antigen (CA) 19-9 greater than 100 U/L. Mean adjusted 5-year survival rates—according to the presence of 0, 1, 2, 3, or 4 of these factors—were 59%, 30%, 7%, 0%, and 0%, respectively (Adam et al, 2004a).
Since those first retrospective reports, prospective trials have provided additional data concerning outcomes of conversion chemotherapy, but few studies report on long-term outcome after resection (Table 87.1). Resectability rates following preoperative chemotherapy for 20 patients presenting with unresectable CLM have been reported as high as 80% (Mentha et al, 2006). Subsequent reports with larger sample sizes provide more modest results with respect to conversion to resectability after chemotherapy (15% to 24%) (Masi et al, 2009; Tournigand et al, 2006). Variability in chemotherapy type, duration, and timing with respect to resection and a lack of standard definitions of resectability make comparison between studies impossible.
Table 87.1 Outcome Associated with Resection Following Conversion Chemotherapy of Initially Unresectable Colorectal Liver Metastases
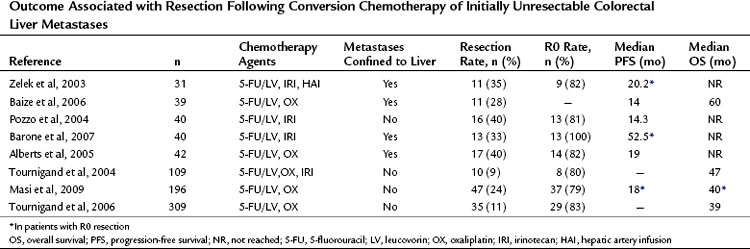
Two recent prospective studies compared long-term outcomes between primary resection and secondary resection following systemic therapy. Capussotti and colleagues (2006) found no statistical difference in overall survival after resection in 34 patients who were converted to resectable, compared with 116 patients who underwent immediate partial hepatectomy (median survival 41 vs. 50 months, respectively; P = NS). At a median follow-up of 35 months, a 94% recurrence rate was observed in the conversion group compared with 66% in the immediate-resection cohort (P = .001). Disease-free survival was 9 months in the converted group compared with 37 months in the immediate-surgery group (P = .001). These authors also noted that extrahepatic recurrence after resection was greater in patients who were converted from unresectable to resectable (Capussotti et al, 2006).
The second study by Nuzzo and colleagues (2007) compared the outcomes of 60 initially resectable patients to that of a cohort of 15 patients with initially unresectable CLM who underwent complete resection following conversion chemotherapy. This study also found no significant difference in overall survival between initially resectable and unresectable patients with a mean survival of 46 and 47 months, respectively (P = NS). At a median follow-up of 34 months, disease recurrence rates were higher in initially unresectable patients (53%) compared with those of the primarily resectable comparison group (28%), and 3-year disease-free survival rates were 31% and 58%, respectively (P = .04) (Nuzzo et al, 2007).
Combining Systemic Therapy with Surgery
Preoperative Versus Postoperative Chemotherapy
The use of adjuvant chemotherapy in patients who have undergone resection of colorectal liver metastases has the theoretic benefit of decreased recurrence rates from addressing potential micrometastases before they become too large to treat medically. Early evidence of the effect of systemic chemotherapy on resected patients was published by Figueras and colleagues in 2001. They reported on a series of 235 patients who underwent partial hepatectomy with curative intent. Of 180 patients considered for adjuvant treatment with six cycles of 5-FU, only 99 were actually treated, and several patients received oxaliplatin in addition. Patients receiving adjuvant therapy had an improved 5-year survival rate of 53%, compared with 25% for those intended to receive therapy but denied treatment, or for those who declined for other reasons (P < .001). Multivariate analysis revealed adjuvant chemotherapy as an independent predictor of survival (Figueras et al, 2001). Selection bias in this report limits interpretation of the results.
The largest retrospective multicenter study from Parks and colleagues (2007) included 247 patients treated with adjuvant 5-FU–based chemotherapy following resection of CLM and 518 patients who received no adjuvant treatment. They found an increase in overall survival with the use of adjuvant chemotherapy with a 47-month median survival and a 5-year survival of 37% compared with 36 months and 31%, respectively, for patients who did not receive chemotherapy (P = .007). Stratifying patients according to the clinical risk scores (Fong et al, 1999), they noted that patients who received adjuvant chemotherapy had improved survival in each category (range, 1.3- to 2.0-fold) (Parks et al, 2007).
Subsequently, Portier and colleagues (2006) conducted a multicenter, randomized, controlled trial that compared surgery combined with adjuvant 5-FU/LV to surgery alone. After recruiting 173 patients who had undergone R0 resection, this trial was aborted early because of low accrual rates. Although the study was underpowered, the investigators reported their results. At a median follow-up of 87 months, they demonstrated an improved 5-year disease-free survival rate in the adjuvant chemotherapy group compared with that of the surgery-alone group (33.5% vs. 26.7%; P = .028); they also found a nonsignificant trend toward increased 5-year overall survival (51.1% vs. 41.9%; P = .13). Recently, Mitry and colleagues (2008) pooled the results of this trial with another trial reported in abstract form to overcome the lack of statistical power. By combining patients, they were able to increase the study population to 278, of which 138 (50%) received six cycles of adjuvant 5-FU/LV following complete resection. In this combined dataset, a trend toward improved median progression-free survival was noted (27.9 months vs. 18.8 months; P = .058) and a trend toward increased overall survival (62.2 months vs. 47 months; P = .095) for the adjuvant chemotherapy group. Treatment with adjuvant chemotherapy was independently associated with improved disease-free and overall survival by multivariate analysis.
Although these data suggest a benefit for adjuvant systemic therapy following hepatic resection for patients with CLM, other investigators have raised the possibility of using a preoperative approach, as has been championed in the treatment of rectal cancer (Bosset et al, 2005; Sauer et al, 2004). The rationale and theoretic benefits of preoperative chemotherapy include the ability to 1) treat micrometastatic disease prior to exposing patients to the regional growth factors that attend surgery following partial hepatectomy; 2) downsize tumors; 3) ensure that patients will get some systemic therapy, as complications after partial hepatectomy may preclude this; and 4) identify those patients who have aggressive tumor biology with disease progression while on systemic therapy, who would not likely have benefited from surgical resection. On the other hand, the risks of preoperative chemotherapy include liver toxicity, increased risk of disease progression or development of new metastatic sites, and selection of more resistant cell clones (Kemeny, 2007).
The best data have been published by the European Organization for Research and Treatment of Cancer (EORTC). Their prospective randomized controlled trail enrolled 364 patients and compared a group who received perioperative FOLFOX and surgery to a group who received surgery alone. A median of six cycles were given prior to and following resection, and all patients had four or fewer lesions and were deemed resectable prior to enrollment. This trial demonstrated a 7.3% progression-free survival benefit, from 28.1% (95.66% confidence interval [CI], 21.3 to 35.5) to 35.4% (CI, 28.1 to 42.7; hazard ratio [HR], 0.79 [0.62 to 1.02]; P = .06) for patients receiving chemotherapy along with surgery (Nordlinger et al, 2008). Only 63% of patients (115 of 151) in the chemotherapy group actually received chemotherapy following surgery, of which only 70% (n = 80) received all six cycles. Thirty-six patients did not receive postoperative chemotherapy, mainly because of patient refusal, perioperative complications, toxic effects of preoperative chemotherapy, or disease progression. Overall, the results of this study support the hypothesis that chemotherapy is beneficial for some patients undergoing partial hepatectomy for CLM, but it does not address the question of whether treating patient with preoperative chemotherapy impacts prognosis.
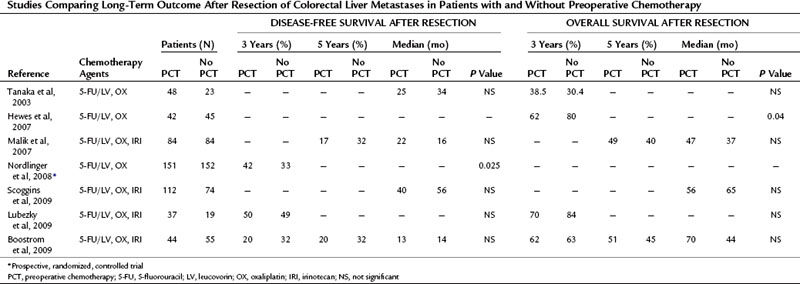
The existing retrospective studies that compare survival after resection of CLM with and without preoperative chemotherapy typically include patients who also received adjuvant chemotherapy. To date none of these studies demonstrates a significant benefit from preoperative chemotherapy (Table 87.2).
Table 87.2 Studies Comparing Long-Term Outcome After Resection of Colorectal Liver Metastases in Patients with and Without Preoperative Chemotherapy
In Vivo Response to Systemic Treatment
It seems clear that chemotherapy improves the results of resection, but it remains unclear whether such therapy is optimally given before or after surgery. One of the potential benefits of administering chemotherapy prior to hepatic resection for CLM is to assess in vivo response of disease. Adam and colleagues (2004b) investigated the response to chemotherapy as a predictor of long-term outcome in 131 patients after potentially curative resection of multiple liver metastases (≥4). After a median of nine courses of 5-FU/LV with either irinotecan or oxaliplatin, they observed objective tumor response in 58 patients (44%), stable disease in 39 (30%), and tumor progression in 34 (26%). Response to chemotherapy correlated with overall survival following hepatectomy at 37%, 30%, and 8%, respectively, at 5 years (P < .001). In another recent retrospective analysis of 88 patients treated with preoperative oxaliplatin or irinotecan-based chemotherapy regimens followed by partial hepatectomy for resectable CLM, Chiappa and colleagues (2009) also noted that response to chemotherapy on restaging was associated with a significantly improved 5-year survival compared with that of nonresponders (71% vs. 15 %; P = .026).
Two studies from Memorial Sloan-Kettering Cancer Center (MSKCC) addressed the question of chemoresponsiveness of CLM and survival in patients who had staged resections. Allen and colleagues (2003) found that the 46 patients who did not progress on chemotherapy administered prior to hepatic resection saw a 5-year survival benefit over that of the 54 patients who did not receive chemotherapy (85% vs. 35%; P = .03). The results of this analysis suggest that a clinical response to chemotherapy can identify a subset of patients with biologically favorable tumors, and treating clinicians can select them for more aggressive therapy. In a separate study by Gallagher and colleagues (2009) of 111 patients from MSKCC who underwent hepatic resection after preoperative chemotherapy, no differences were seen in median overall survival among patients who responded, had stable disease, or showed progression of disease (58 months vs. 65 months vs. 61 months; P = .98); however, it should be noted that the protocol for this evaluation was to proceed with hepatectomy at the first clinical sign of progression, as opposed to proceeding with resection at the first sign of response.
It remains unclear what the optimal duration of preoperative chemotherapy should be and when to proceed with hepatic resection; however, more surgeons are advocating proceeding with hepatic resection at the first signs of tumor response to avoid extended exposure of the liver to cytotoxic chemotherapy (see Chapter 65). To address this question, White and colleagues (2008)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







