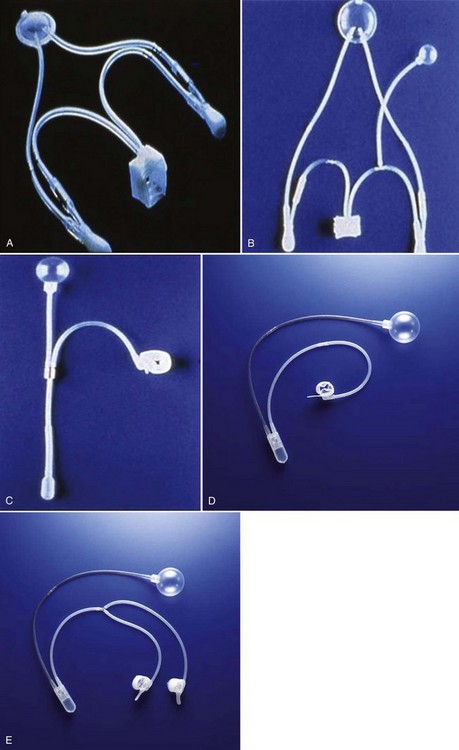
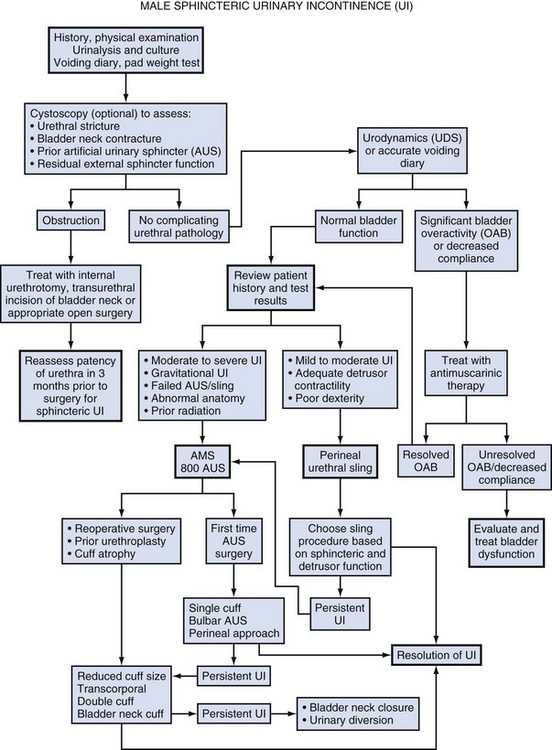
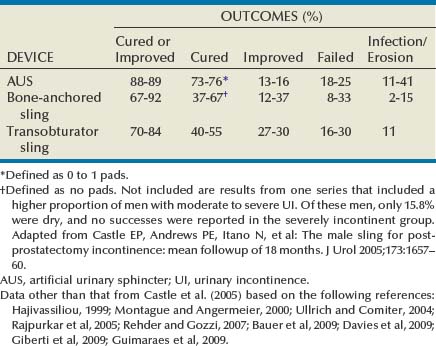
Hunter Wessells, MD, FACS, Andrew C. Peterson, MD, FACS Urinary incontinence (UI), the complaint of any involuntary leakage of urine (Abrams et al, 2002), may be the result of congenital anomalies, injury, genitourinary surgery, and other conditions. The most common etiology of sphincteric UI in men is radical prostatectomy (RP), with the primary mechanism being failure to store urine secondary to inadequate resistance of the outlet sphincter. Despite improvements in surgical technique that have reduced the rate of post-prostatectomy UI, the burden of disease in the United States remains high and is expected to rise because of increasing numbers of RP performed annually (Stanford et al, 1999). UI significantly compromises heath-related quality of life in men (Coyne et al, 2003) and can be improved by surgical treatment, including transurethral bulking agents, bulbar urethral slings, and the artificial urinary sphincter (AUS). These procedures prevent involuntary urinary loss by increasing outlet resistance. Although bulking agents have been used in the past as first-line treatment for male sphincteric UI, the severity of incontinence and postsurgical scarring in the vesicourethral region postprostatectomy have made surgical correction first-line treatment for the majority of cases. Urinary continence in the male depends on a compliant and contractile bladder body, functional posterior urethra, including the bladder neck and prostate (internal sphincter) and an intact rhabdosphincter (external sphincter). Thus stress urinary incontinence develops only in men with concomitant internal and external sphincter impairment. Internal sphincter incompetence results from pelvic surgery, bladder neck injury, specific sympathetic neuropathic dysfunction, or embryologic disruption. Incompetence of the external sphincter, known as intrinsic sphincter deficiency (ISD), occurs most frequently after radical prostatectomy, but also can result from prostatomembranous urethral distraction injuries, traumatic and acquired myelopathy, and congenital disorders, such as spinal dysraphism, sacral agenesis, and the exstrophy/epispadias complex. Table 79–1 shows conditions in which the bladder neck and rhabdosphincter may be dysfunctional or compromised. Associated bladder dysfunction, including decreased compliance or detrusor overactivity, may complicate or confound the diagnosis and management of male UI and requires careful evaluation (see below). Male incontinence is rare in the general population, affecting only 5% of older men (Bortolotti, 2000). The incidence of UI in men parallels the rate of various surgical procedures and urologic conditions listed in Table 79–1. Incontinence after transurethral prostatectomy (TURP) may reflect persistent bladder overactivity but rarely results from damage to the external sphincter during transurethral resection. In a large Veterans Affairs Cooperative Study (Wasson et al, 1995), the rate of de novo UI after TURP was no different than in the watchful waiting group. The historical incidence of incontinence after radical prostatectomy varies from 2.5% to 87% (Foote et al, 1991). Progressive improvement in urinary control has been reported to occur for as long as 2 years after surgery; in one study the percentage of men who used one pad or less after RP was 71%, 87%, 92%, and 98.5% at 3, 6, 12, and 24 months, respectively (Lepor and Kaci, 2004). Nerve-sparing techniques pioneered by Walsh (Eggleston and Walsh, 1985) have been associated with a reduced incidence of postoperative UI (Wei et al, 2000a). Although the mechanism of this effect remains debatable, sensory and motor pudendal innervation of the rhabdosphincter is generally preserved post-RP; in contrast, autonomic afferent denervation and impaired membranous urethral sensitivity seems to be associated with post-RP UI (Catarin et al, 2008). Bladder neck contracture is also associated with a reduced continence rate after RP, probably due to secondary surgical intervention (Park et al, 2001). Contemporary series demonstrate improved continence after prostatectomy when compared with historical rates, but wide variation in reported outcomes reflect patient- and surgeon-specific factors as well as methodology of data collection (Flynn and Webster, 2004). Validated disease-specific questionnaires, such as the University of California–Los Angeles (UCLA) Prostate Cancer Index (Litwin et al, 1998) or the Expanded Prostate Cancer Index Composite (Wei et al, 2000b), that capture symptoms of stress and urgency incontinence are recommended. Rates of incontinence are higher when calculated from such self-report instruments as compared with chart review or physician-recorded outcomes (Carlson and Nitti, 2001; Flynn and Webster, 2004). For example, Litwin and colleagues (1995) reported that up to 40% of patients in a large cohort study complained of persistent long-term urinary incontinence after RP. Although most patients reported mild UI, 4% complained of significant lifestyle-compromising leakage. Devices to control urinary incontinence in men have been described since antiquity (Schultheiss et al, 2000). One of the first modern prosthetic devices to treat incontinence in men was reported by Berry and associates in 1961 (Engel and Wade, 1969). This acrylic prosthesis was designed to kink and compress the bulbar urethra, but poor results, pain, and fistula formation led to its abandonment (Engel and Wade, 1969). In 1970, Kaufman reported the first of several procedures to provide continence by compressing the urethra with the Kaufman I procedure. This was modified by incorporating a tetrafluoroethylene mesh tape in the Kaufman II procedure and a silicone-gel–filled hemispherical prosthesis in the Kaufman III procedure, giving a success rate of 70%. Slings to treat stress urinary incontinence (SUI) in men were introduced in 1975 by Kishev, who described the placement of a pliable prosthetic “wad” under the bulbar urethra. Schaeffer then described a bulbourethral sling that used bolsters suspended from the rectus fascia (Schaeffer et al, 1998; Clemens et al, 1999). Another report documented results with a composite graft of polypropylene and porcine skin collagen placed suburethrally (John, 2004). Male bulbourethral slings have continued to evolve with the development of bone screws for synthetic mesh fixation (InVance; American Medical Systems, Minnetonka, MN), transobturator fixation (AdVance; American Medical Systems), and a newly announced combined prepubic and transobturator sling (Virtue; Coloplast, Minneapolis, MN) (Comiter and Rhee, 2008). In 1976, Rosen designed the first model of an AUS, but hydraulic failure and fistula formation approached 100%, and this device was abandoned (Fowler and Auld, 1985). Scott presented his initial report with the AMS 721 (American Medical Systems, Minnetonka, MN), noting a 79% success rate (Timm et al, 1976; Scott, 1978). Over the next 10 years, further developments in design of the AUS were made. The AMS 742 allowed automatic cuff closure after cuff decompression. The AMS 791 and 792 used a silicone rubber cuff and a deactivation button (Scott, 1989). The AMS 800 included the deactivation button within the control pump; and, in 1987, the narrow-backed cuff was introduced (Fig. 79–1), which, by improving pressure transmission from the cuff pressure to the underlying tissue, has greatly decreased the incidence of urethral erosion and tissue atrophy (Light and Reynolds, 1992). The initial evaluation of a man with UI requires a detailed history, physical examination, and urinalysis. Many cases of sphincteric UI will also require cystoscopy and pressure flow urodynamics to evaluate potential bladder neck contracture and bladder storage function respectively. Figure 79–2 shows the overall management algorithm. The medical history should focus on the type, degree, and severity of UI; previous surgical procedures; and symptoms of neurologic disease. Specific evaluation of the failed AUS will be considered at the end of this section. Differentiation between stress and urge UI is important and can be aided by the voiding diary and pad test, simple inexpensive assessments of urinary incontinence that are recommended before proceeding with invasive testing (Flynn and Webster, 2004). The voiding diary reliably assesses the number of incontinent episodes and may uncover significant urgency and urge incontinence (Groutz et al, 2000). Self-reported daily pad usage varies considerably, with only moderate concordance with urinary incontinence volume (Dylewski et al, 2007). Thus the 24-hour pad weight test, which objectively measures the magnitude of the incontinence, may be helpful in directing appropriate therapy. However, because the effectiveness of current surgical therapies has not been stratified according to severity of UI, the most important current use for the pad test may be as an outcome measure postoperatively. Detection of vesicourethral anastomotic stricture, bladder neck contracture, and bulbar urethral stricture prior to planned surgery may be achieved by history or uroflowmetry in most cases (Yurkanin et al, 2001). Because unrecognized urethral pathology can significantly complicate all surgical approaches, endoscopic evaluation is recommended prior to surgical correction of UI post-RP or -TURP even though bladder neck contracture is rare (Wasson et al, 1995; Erickson et al, 2009; Krambeck et al, 2009). Cystoscopic evaluation of the degree of residual function of the external sphincter may help identify appropriate candidates for transobturator sling procedures (see section on Transobturator Bulbourethral Sling and Fig. 79–3). Furthermore, in patients with recurrent incontinence after AUS implantation, cystoscopy may aid in differentiating mechanical failure from other causes such as urethral atrophy. Finally, when an AUS or sling (Harris et al, 2009) has been removed due to infection or erosion, repeat evaluation of the urethra prior to reimplantation is essential to identify stricture, diverticulum, and other urethral complications. Assessment of bladder capacity, compliance, and contractility is required prior to considering surgical correction of UI. A careful history and voiding diary may be sufficient to confirm the adequacy of bladder function; however, pressure-flow urodynamics permits an accurate assessment of bladder function, incontinence type, and severity. ISD will be identified in almost all cases. Although 40% to 45% of men with UI post-RP have bladder dysfunction, it is the sole cause of incontinence in a very small percentage (Leach et al, 1996; Ficazzola and Nitti, 1998). Filling cystometry can be difficult in men with severe incontinence, and occlusion of the bladder neck with a balloon catheter may be required to assess compliance and overactivity. Detrusor overactivity, although not a contraindication to surgery for UI if discovered, requires realistic counseling regarding the likelihood of a successful outcome. Reduced bladder compliance presents a more serious concern, because prolonged storage at high pressures may lead to deteriorating renal function. Notably, such urodynamic findings do not reliably predict worse post-AUS continence (Trigo-Rocha et al, 2008; Lai et al, 2009). This effectiveness, in spite of adverse urodynamic findings, could portend silent upper tract deterioration. Conversely, detrusor hypocontractility on pressure-flow urodynamics may indicate the need for AUS, if adequate detrusor function does not exist to overcome the fixed resistance of a compressive sling (Comiter, 2007). The diagnostic evaluation of recurrent UI after AUS must differentiate between inadvertent deactivation, insufficient urethral compression (oversizing of cuff), mechanical failure with fluid loss, cuff erosion, bladder storage failure, urethral or bladder neck atrophy under the cuff, as well as rare causes such as a plugged delay-fill resistor or kinked tubing (Montague and Angermeier, 2001). The evaluation includes the same general approach described for de novo UI in Figure 79–2. A history of sudden loss of continence suggests deactivation or mechanical failure. Active cycling of the device excludes inadvertent deactivation. If the pump is deactivated with inadequate fluid to cycle, passive filling can be achieved by squeezing the pump on its lateral edges or by pushing on the pump with a cotton-tipped applicator opposite the deactivation button. Fluid loss from the device implies mechanical failure and leak. Plain radiography (for contrast-filled systems) or ultrasonography (for saline-filled systems) of the PRB during cycling can help differentiate fluid loss from cuff atrophy. If the PRB size changes with cycling and refills passively, mechanical failure is less likely, thus suggesting cuff atrophy (Taylor and Lebowitz, 1985; Lorentzen et al, 1987). Cystoscopy, in addition to excluding erosion, can be used to visualize the cuff during cycling and give insight into the likelihood of atrophy. Urodynamics should be performed when bladder storage failure is suspected. Key Points: Initial Evaluation Surgical correction of UI is indicated in male patients with irreversible intrinsic sphincter deficiency and bothersome involuntary leakage of urine. After prostatectomy, all men should undergo a course of pelvic floor muscle exercise. Because progressive improvement in continence occurs after RP, some authors recommend a 1-year observation period (Peyromaure et al, 2002; Flynn and Webster, 2004). However, it is unnecessary to delay intervention for patients with severe or gravitational UI who show no improvement beyond 6 months, particularly if cystoscopy shows a significant external sphincter defect. Because of limited efficacy, submucosal bulking agents are no longer part of the treatment algorithm for post-RP UI (Kuznetsov et al, 2000; Montague and Angermeier, 2000; Abrams et al, 2009). Thus AUS and slings should be recommended as first-line surgical therapy for sphincteric incontinence in most men, although a trial of bulking agent may be appropriate in cases of neurogenic male stress UI. Once the diagnostic evaluation has been completed (see Fig. 79–2), the best option for a given patient can be selected. Factors to consider include the severity of UI and associated bother; patient characteristics, including prior surgical procedures, bladder function, and cystoscopic findings; manual dexterity and cognitive function; efficacy of the various implants; long-term risk of complications and reoperation; and patient preference. Absolute contraindications to surgical correction of male UI are few, and include bladder disorders that jeopardize renal function, such as diminished vesical compliance and vesicoureteral reflux at low intravesical pressure. Inadequate tissue integrity at the bladder neck or urethra to accommodate a sling or AUS may require bladder neck closure or urinary diversion. Additionally, urinary tract abnormalities that require future transurethral management, such as bladder cancer or refractory vesicourethral anastomotic strictures, should be considered relative contraindications to surgery. In such cases, an AUS or sling procedure could impair transurethral access, and repeated instrumentation may put the devices at risk for infection or erosion. Although metastatic prostate cancer is not a contraindication to surgical correction of UI, improvements in quality of life must be balanced against performance status and life expectancy. The AUS remains the established device for treatment of moderate to severe UI, supported by numerous publications documenting its benefits (Table 79–2). Advantages include reproducible and reliable implantation, ability of patients to empty the bladder without detrusor contraction, and proven efficacy after pelvic irradiation. A national study using validated questions from the University of California–Los Angeles Prostate Cancer Index (UCLA PCI) provides useful data on AUS results (Dalkin et al, 2003) (Table 79–3). Few men post-AUS have severe degrees of incontinence (9%), but many continue to use pads and report a moderately high urinary bother. There is accumulating data that men who are completely dry after radical prostatectomy have significantly better health-related quality of life compared with those who wear one pad daily (Cooperberg et al, 2003). Whether this is also the case after AUS remains unknown. Long-term durability of the AUS is well established, although a revision rate of 16% and 28% at 2 and 5 years, respectively highlights the limitations of the devices (Dalkin et al, 2003) and is consistent with a large single institution series in which 72% had the original sphincter in place and functioning at a mean follow-up of 69 months (Elliott and Barrett, 1998). Table 79–3 National Questionnaire-Based Assessment of AUS Outcomes 2 and 5 Years after Implantation Age-adjusted urinary function and bother scores derived from the UCLA PCI. AUS, artificial urinary sphincter; UCLA PCI, University of California–Los Angeles Prostate Cancer Index. * Results are expressed as a numerical score (mean and SD) ranging from 0 to 100, with 100 being the highest possible response. Adapted from Dalkin BL, Wessells H, Cui H: A national survey of urinary and health related quality of life outcomes in men with an artificial urinary sphincter for post-radical prostatectomy incontinence. J Urol 2003;169:237–9. Table 79–2 Outcomes and Complication Rates of Surgical Therapy for Male Sphincteric Urinary Incontinence Male sling procedures represent a significant addition to the surgical armamentarium for UI by providing an alternative to AUS. They impart outlet resistance without occlusion of the urethra with a cuff, potentially reducing the risk of erosion and mechanical failure (Comiter, 2007). The bone-anchored sling is performed through a single perineal incision that results in short operative times and low erosion rates (Madjar, 2001a; Comiter, 2002). Titanium screws provide stable fixation of the sling to the inferior pubic ramus, avoiding the need for retropubic passage of suspension sutures. The mechanism of continence with the bone-anchored sling relies on compression of the urethra, as demonstrated by an increase in fixed resistance of the urethra (Ullrich and Comiter, 2004). However, no urodynamic evidence of increased detrusor pressure during voiding was seen. The results with this technique have been generally positive, although longer-term follow-up has revealed cases of persistent pain, infection and erosion (see Table 79–2) (Giberti et al, 2009). A transobturator sling introduced by AMS (AdVance) has been shown to be effective in short-term follow up of well-characterized case series (see Table 79–2) (Rehder and Gozzi, 2007; Davies et al, 2009). In these highly selected populations, approximately 75% of patients are cured or significantly improved, with results persisting at 12 months. Unlike the bone-anchored sling, in which continence is improved by compression, it appears that the transobturator sling augments sphincteric function by repositioning and lengthening the membranous urethra (Firrozi and Vasavada, 2009). Although the absence of increased detrusor pressure during voiding was interpreted as a noncompressive mechanism (Davies et al, 2009), the significant tensioning required during sling placement suggests that compression may play a role in the function of the transobturator sling as well (Latini, 2009). Bulbar urethral slings can be seen as alternatives for those who refuse AUS from fear of infection, erosion, or mechanical failure, as well as those with limited physical or cognitive capacity. Comiter (2007) notes that the techniques differ in their indications, relative success rates, and complication rates for patients with varying degrees of incontinence. The trade-off between risk and efficacy most be considered, with most authors recommending AUS for more severe UI. For mild UI, bulbar sling procedures become viable alternatives, whereas AUS may represent therapeutic overkill. Thus the bone-anchored and transobturator slings should be primarily used in cases with mild incontinence, which can be defined as a 24-hour pad weight of less than 150 grams (Flynn and Webster, 2004). A sling procedure should not be offered to those with prior radiation therapy or urethral erosion, because the degree of urine loss that exists in this group usually exceeds the limits of the procedure (Schaeffer et al, 1998; Dikranian et al, 2004; Castle et al, 2005; Giberti et al, 2009).
Classification, Pathophysiology, and Etiology
History and Development of Devices
Evaluation and Diagnosis
History
Cystoscopy
Urodynamics
Evaluation of Persistent Incontinence after AUS
Indications for Surgery
2 YEARS POST-AUS
5 YEARS POST-AUS
Category
Urinary function*
53 (25)
40 (22)
Urinary bother*
59 (24)
48 (32)
Degree of Incontinence (%)
Mild
59
38
Moderate
32
37
Severe
9
24
Revision Rate (%)
16
28
N
289
292
Surgical Procedures for Sphincteric Incontinence in the Male: The Artificial Genitourinary Sphincter and Perineal Sling Procedures
• A careful history and voiding diary characterizes the type and severity of urinary incontinence in most men.