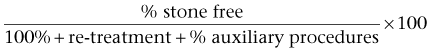Brian R. Matlaga, MD, MPH, James E. Lingeman, MD Although calculi in the kidney were rare before the Industrial Revolution (Shah and Whitfield, 2002), the existence of nephrolithiasis was known to Hippocrates, who described the symptoms of renal colic: “An acute pain is felt in the kidney, the loins, the flank and the testis of the affected side; the patient passes urine frequently; gradually the urine is suppressed. With the urine, sand is passed.” It is not certain whether Hippocrates actually performed surgery on patients with renal calculi, but he did describe the following operations: the drainage of tuberculous and nontuberculous pyelonephritic abscesses, the incision of swelling in the loin due to renal tumefaction resulting from stone, and the drainage of kidneys presenting with acute congestion caused by pyelonephritis (Wershub, 1970). In the centuries that followed Hippocrates there was little scientific progress in the surgical therapy for patients with renal calculi. The alleged first account of a surgical attempt to remove a stone from a patient’s kidney is the case of the French archer of Bagnolet. Little is known of the authenticity of this tale of a condemned man afflicted with a renal calculus who agreed to allow surgery on the affected kidney with the condition that if he survived he would be freed. According to the anecdote the man survived the open surgical stone removal and was freed in 1474 (Herman, 1973). Unfortunately there are no first-hand records of this event so the veracity of this claim is uncertain. The first verifiable account of renal stone surgery was in 1550, when Cardan of Milan opened a lumbar abscess on a young girl and removed 18 calculi (Desnos, 1972). For the following 2 centuries most surgeons were in agreement that the only indication for open renal surgery was the infected calculous kidney, distended by the accumulation of purulent matter, or those kidneys in which the calculus could be palpated in the organ itself. In 1734 Lafite incised a swelling in a patient’s loin and drained considerable purulence. Twenty-two days later the pus reaccumulated; he probed the incision and found a stone in the region of the kidney. Lafite widened the prior incision and removed two calculi; the patient recovered well. Four years later Lafite again removed stones from a man who had undergone drainage of a lumbar swelling 11 years before and who had a persistent urinary fistula. Lafite concluded that it was possible to remove the stones at the time of the first surgical intervention rather than subject the patient to multiple procedures (Ballenger et al, 1933). In 1872, William Ingalls of Boston City Hospital removed a large calculus from the right kidney of a 31-year-old woman with a persistent pyelocutaneous fistula (Spirnak and Resnick, 1983). Ingalls incised the sinus tract of the fistula and extracted the stone with forceps, thus performing the first recorded nephrolithotomy in America. In 1880, Henry Morris of England was the first to remove a stone from an otherwise healthy kidney by nephrolithotomy, extracting a 31-g mulberry calculus from the kidney of a young woman (Dudley, 1973). As the surgical techniques of nephrolithotomy evolved, renal parenchymal incisions were made in a variety of different ways in an effort to reduce hemorrhagic morbidity. Heineke, in 1879, first described a pyelotomy incision for the extraction of calculi. The operation rapidly found favor and was employed by many surgeons, although it was not possible to extend the incision to permit extraction of large renal calculi without damaging the retropelvic renal artery (Wershub, 1970). Josef Hyrtl, in 1882, and Max Brödel, in 1902, described a relatively avascular plane near the midline (5 mm posterior) of the convex border of the kidney through which the collecting system of the kidney could be entered. In continental Europe, credit for the plane was given to Hyrtl; but in England and the United States it was called the Brödel bloodless line or the Brödel white line (Schultheiss et al, 2000). Although the existence of this avascular plane was an important discovery, surgeons continued to find that bleeding during nephrolithotomy was a considerable problem. Zuckerkandl described an inferior pyelonephrolithotomy in which a pyelotomy incision was extended into the lower pole of the kidney. Partner recommended a V-shaped incision with two limbs radiating toward the poles of the kidney. Other attempts were made to control the persistent problem of bleeding, including compression of the hilar vessels and various methods of suturing. In 1887, Czerny was the first to approximate the cut edges of the incised kidney with suture to control hemorrhage and to prevent fistula formation. In the same year, Guyon reported that nephrectomy, although efficacious in curing patients suffering from calculous pyonephrosis, was more dangerous than nephrolithotomy because lithiasis was often bilateral (Wershub, 1970). In 1889 Kümmell was the first surgeon to perform a partial nephrectomy for calculous pyonephrosis (Redman, 1983). Lower, in 1913, revived interest in pyelolithotomy when he suggested that this technique may be a safer and easier method of removing renal calculi than nephrolithotomy. Although several small series of cases indicated that there might be a higher incidence of stone recurrence after pyelolithotomy, other studies showed that recurrence was no more common than it was after nephrolithotomy (Murphy, 1972). These findings, in conjunction with rapid advancements in the field of radiography, brought about a decided preference for pyelolithotomy (Gil-Vernet and Culla, 1981). In 1943 Dees and Fox reported the first use of coagulum to remove small stones and stone fragments from the renal pelvis and calyces (Marshall, 1983). Fibrinogen and thrombin were used to make a coagulum that was injected into the renal pelvis and produced a flexible cast of the pelvis and calyces. The use of this technique was limited initially owing to the scarcity of materials and the risk of blood-borne disease transmission. However, interest in coagulum pyelolithotomy was renewed when cryoprecipitate was found to be a safe and readily available source of concentrated fibrinogen (Fischer et al, 1980). An important advance in the open surgical approach to the kidney was the intrasinusally extended pyelolithotomy, pioneered by Gil-Vernet in 1965. Because of its wide applicability and minimal morbidity this approach to the renal collecting system became the procedure of choice for treatment of the majority of renal pelvic calculi. Patients harboring large or complex calculi could be effectively treated with extended pyelolithotomy combined with multiple radial nephrotomies (Wickham et al, 1974). In 1968 Smith and Boyce described anatrophic nephrolithotomy, a procedure that derived its name from the technique of incising the renal parenchyma along the avascular plane between the anterior and posterior vascular distributions. Because an incision in this plane does not interrupt the blood supply to the renal parenchyma it does not result in atrophy, hence the term anatrophic. This procedure permits a relatively bloodless operation that encompasses stone removal, reconstruction of the calyceal system, and closure of the renal capsule with preservation of renal function. Although stone-free rates of these modern surgical techniques were excellent, morbidity was significant, and the search for new techniques and technologies continued. Ambroise Paré is credited with the first account of a ureteral calculus, when, in 1564, he described “the cruel pain [that] tormented the patient in that place where the stone lodged.” Paré also stated that death was the consequence of having calculi impacted in both ureters (Murphy, 1972). Morris recounted that surgical intervention was an option in the treatment of ureteral stones when he reported in 1898 that “operations on the ureter are an advance of the last few years, but not many have been recorded up to the present time” (Ballenger et al, 1933). Thomas Emmet, of New York, published an account in 1879 of three female patients with stones impacted at the distal aspect of the ureter. In one patient Emmet opened the bladder and removed the stone with forceps; in a second patient he removed a stone by cutting down on it through the vaginal wall. These procedures were the first records of a surgeon making a definite diagnosis of ureteral calculus and deliberately and successfully performing a ureterolithotomy. In the years that followed, intraperitoneal, perineal, sacral, transrectal, and transvaginal approaches were used. In 1910 Gibson, of New York, described an incision parallel to and just above the Poupart ligament, wholly extraperitoneal, by which the lower ureter, even down to its entrance into the bladder, could be readily exposed. This safe and comparatively easy approach to the ureter gave open ureterolithotomy a solid basis for success. Before the development of endoscopy attempts to blindly extract calculi were not uncommon. In 1889, Gustav Kolisher performed the first successful stone manipulation, reporting that he “located the stone with a metal-tipped catheter several inches above the ureteric orifice and through it injected 30 cc of sterile oil,” displacing the stone (Murphy, 1972). The development of minimally invasive surgical techniques for the treatment of patients suffering from urinary lithiasis has been greatly dependent on technologic advances in the fields of fiberoptics, radiographic imaging, and lithotripsy (shockwave, ultrasonic, electrohydraulic, and laser). These advancements have accelerated the evolution of modern techniques of calculus removal, including ureteroscopy, percutaneous nephrolithotomy (PNL), and extracorporeal shockwave lithotripsy (SWL). In 1979 Arthur Smith defined the term endourology as closed controlled manipulation within the genitourinary tract (Smith et al, 1979). The practice of ureteroscopy began by happenstance when, in 1912, Hugh Hampton Young introduced a pediatric cystoscope into the massively dilated ureter of a child with posterior urethral valves (Young and McKay, 1929). Aided by the child’s secondary ureteral dilation, Young was able to advance the cystoscope to the level of the renal pelvis, thus becoming the first urologist to view the intrarenal collecting system endoscopically. Unfortunately the following 3 decades held few significant advances in ureteroscopic technology until knowledge of fiberoptics could be put to clinical use. By 1957 Curtiss and Hirschowitz combined a large number of glass fibers into a coherent bundle and fused the fibers at their ends to allow them to move individually along their length, thus creating the first flexible endoscope (Hirschowitz et al, 1957). In 1964 Marshall reported the first urologic use of this new type of flexible endoscope when he passed the scope through an open ureterotomy to the level of the renal pelvis, thereby performing the first flexible ureteroscopy. Subsequently, two of his associates, McGovern and Walzak, performed the first transurethral flexible ureteroscopy when they passed the same 9-Fr flexible endoscope to inspect a ureteral calculus. Since then, developments in optics and mechanics have greatly improved the design of flexible ureteroscopes. More recently, efforts have been devoted to advancing the imaging capability of the flexible ureteroscope. Digital endoscopes, which incorporate an optical chip [complementary metal oxide semiconductor [CMOS] or charge-coupled device [CCD]) at the tip have been introduced. Although the initial generation of these chips were quite large, further refinements have reduced their size so that they can be applied to flexible ureteroscopes. Advantages associated with this technology include improved optical characteristics, obviating focus and white-balancing issues, and decreased surgeon fatigue, because cumbersome proximal camera and light cord attachments are not required. Image processing software permits digital zoom capability as well. In comparing digital flexible ureteroscopy to conventional fiberoptic flexible ureteroscopy, investigators have noted that the digital devices are associated with superior imaging characteristics (Humphreys et al, 2008). Several disadvantages of the digital device must be recognized, however: digital ureteroscopes are larger in diameter compared with their fiberoptic counterparts, and the technology also is more costly. Interestingly, the first reports of rigid ureteroscopy trailed those of flexible ureteroscopy by almost 10 years. In 1977 Goodman reported on three cases in which a pediatric cystoscope was used to treat patients with ureteral maladies. These initial rigid ureteroscopes employed a rod-lens system that was large (10 to 13 Fr) and inflexible. Most rigid ureteroscope designs have replaced this rod-lens system of image transmission with fiberoptics, which allows significant reduction in the size of the endoscope. In addition, the flexibility of the fiberoptic bundles allows the shaft of the endoscope to become somewhat bendable along its vertical axis, hence the term semirigid ureteroscope. The first description of percutaneous stone removal was that of Rupel and Brown (1941) of Indianapolis, who removed a stone through a previously established surgical nephrostomy. It was not until 1955, however, that Goodwin and associates described the first placement of a percutaneous nephrostomy tube to drain a grossly hydronephrotic kidney. These researchers did not have the benefit of radiographic guidance, and so the drainage tube was placed without imaging. In 1976, Fernstrom and Johannson first reported the establishment of percutaneous access with the specific intention of removing a renal stone. Subsequent advances in endoscopes, imaging equipment, and intracorporeal lithotripters allowed urologists and radiologists to refine these percutaneous techniques through the late 1970s and early 1980s into well-established methods for removal of upper urinary tract calculi. High-energy shockwaves, too, have been recognized for many years. Examples of high-energy shockwaves include the blast effect associated with explosions, as well as the potentially window-shattering sonic boom created when aircraft pass beyond the speed of sound. Engineers at Dornier Medical Systems in what was then West Germany, during research on the effects of shockwaves on military hardware, demonstrated that these shockwaves are reflectable and therefore focusable. The possibility of applying shockwave energy to human tissue was discovered when, by chance, a test engineer touched a target body at the very moment of impact of a high-velocity projectile. The engineer felt a sensation similar to an electric shock, although the contact point at the skin showed no damage at all (Hepp, 1984). This observation and its potential military applications led Dornier to pursue a method of generating a reproducible shockwave. In 1972, on the basis of preliminary studies performed by Dornier Medical Systems, an agreement was reached with Egbert Schmiedt, director of the urologic clinic at the University of Munich, to proceed with further investigation of the therapeutic potential of this technology (Chaussy and Fuchs, 1986). This research was supported by the West German Federal Ministry of Research and Technology, and the development of the Dornier lithotripter progressed through several prototypes, ultimately culminating in February 1980 with the first treatment of a human by SWL. The production and distribution of the Dornier HM3 lithotripter began in late 1983, and SWL was approved by the U.S. Food and Drug Administration in 1984. Since Dornier’s pioneering work, numerous other companies have demonstrated that shockwaves capable of stone fragmentation may be generated by electromagnetic induction, microexplosions, focused lasers, and piezoelectric crystals. To date, more than 3000 lithotripters of all types have been placed worldwide, and more than 1 million patients are treated annually with SWL. Stone-related factors (size, number, location, composition), renal anatomy, and clinical factors of the patient (Table 48–1) as well as the morbidity inherent in each treatment modality and the availability of the requisite equipment should all be considered before selecting the optimal therapy. Although treatment decisions are ultimately the result of an integrated analysis of a multiplicity of factors, for the sake of simplicity these factors and considerations in management of kidney calculi are reviewed separately (Fig. 48–1). Table 48–1 Factors Affecting Management of Renal Stones (Adapted from Wen CC, Nakada SI. Treatment selection and outcomes: renal calculi. Urol Clin North Am 2007;34[3]:409–19.) The evaluation of patients with urolithiasis in the current era of minimally invasive therapies has not changed substantially from that of the previous era of open stone surgery. Although standard imaging of urinary tract calculi has historically relied on plain abdominal radiography and intravenous urography, recent evidence suggests that unenhanced helical computed tomography (CT) has gained widespread acceptance (Heidenreich et al, 2002). Nephrotomography, radionuclide studies, and retrograde or contrast-enhanced studies are only occasionally necessary to obtain more detailed anatomic and functional information. Bacteriologic evaluation of the urine is mandatory for all patients. The composition of any previous stone material passed or removed from the patient is extremely important. If previous stones have contained significant amounts of calcium oxalate monohydrate (whewellite) or brushite, fragmentation with SWL may be expected to be more difficult. If a stone of such composition is of a large size, the patient may achieve a better outcome with a percutaneous or ureteroscopic procedure rather than with SWL. Cystinuria may be revealed by previous stone analysis or by the characteristic cystine crystals on urinalysis. Any patient whose stone or stones have radiographic features suggestive of cystine (low radiodensity, ground-glass appearance, smooth edges, bilateral stones) should be screened for cystinuria before treatment because these stones are often not well fragmented by SWL. Many stones may harbor bacteria even though bacteriuria is only intermittently present. This is particularly true in the patient who has received antibiotics in the past. Mariappan and associates (2005) have reported that the best predictor of post-PNL urosepsis is stone culture or renal pelvic urine culture results rather than bladder urine culture results. The fragmentation of stones, despite sterile urine, may release preformed bacterial endotoxins and viable bacteria that place the patient at risk for septic complications (Scherz and Parsons, 1987; McAleer et al, 2002, 2003; Paterson et al, 2003). In a review of a large series of patients undergoing PNL, de la Rosette and coworkers (2008) also confirmed that a positive preoperative urine culture was a significant predictor of postoperative morbidity. Therefore, patients who have radiographic or clinical features suggestive of struvite or in whom infection is suspected should receive a regimen of broad-spectrum antibiotics before surgery to reduce the risk of sepsis. Parenteral antibiotics should be administered perioperatively in any patient in whom urinary infection is suspected. Hubner and Porpaczy (1990) reviewed the natural history of calyceal stones followed for an average of 7.4 years. During the observation period, 45% of the stones increased in size, 68% of the patients experienced symptoms of infection, and 51% of the patients experienced pain. Inci and associates (2007) also found that for patients with asymptomatic lower pole calculi, one third of the stones progressed in size and 11% ultimately required surgical intervention. Thus, most calyceal stones, in the absence of intervention, are likely to increase in size and cause symptoms of pain or infection. Furthermore, as time progresses and stone size increases the likelihood of spontaneous stone passage becomes further reduced. Burgher and associates (2004) reported a series of 300 patients initially presenting with asymptomatic renal calculi who were observed for a mean of 3.26 years. Seventy-seven percent of patients experienced progression of calculi, with 26% requiring surgical intervention. Those patients who initially presented with calculi larger than 4 mm were more likely to fail observation than were patients with smaller solitary calculi. Keeley and colleagues (2001) reported the results of a randomized prospective trial of SWL versus observation for 200 patients with small asymptomatic calyceal calculi. Although the authors found little difference in the number of patients in each group requiring additional treatment, the interventions in the observation group were more invasive. Patients in the SWL group required no invasive treatment on follow-up and could be adequately managed with analgesia or antibiotics. However, there was no evidence of a difference in stone-specific symptoms, quality of life, or renal function tests between the two groups at study’s end. The authors concluded that SWL does not appear to improve the clinical outcome of patients with small, asymptomatic renal calculi. Considering the entirety of the literature to the present time, the necessity of treating patients with small (<5 mm), nonobstructive, asymptomatic stones in a prophylactic fashion remains undetermined. However, should asymptomatic stones not be treated, patients must be advised about the need for regular follow-up; a significant proportion of these calculi will eventually become symptomatic and require intervention. Treatment decisions in these situations should be based on the individual patient’s risk factors and the patient’s preference. In several groups of patients, including pediatric patients, patients with a solitary kidney, patients in high-risk professions (e.g., pilots), and women considering pregnancy, treatment of asymptomatic calyceal stones may be indicated. Importantly, if asymptomatic stones are to be managed expectantly, a metabolic evaluation should be performed, to direct a regimen that will prevent stone growth and thereby reduce the need for future surgery (Galvin and Pearle, 2006). Historically, simple calyceal stones documented to be immobile and not causing obstruction have been thought to be unlikely causes of flank pain. However, it has been reported that the pain induced by nonobstructive calyceal stones is characterized by a dull, deep ache, different from the classic pain of renal colic (Coury et al, 1988). It has been reported that endoscopic treatment of calyceal calcifications is associated with improved pain relief for many patients (Taub et al, 2006). Therefore, a patient who is thought to be symptomatic from a calyceal stone should be treated. Staghorn calculi are those stones that fill the major part of the renal collecting system. Typically, they occupy the renal pelvis and branch into most of the calyces, mimicking the horns of a deer or stag (Fig. 48–2). Most staghorn stones are composed of struvite (Segura et al, 1994). Until the early 1970s some physicians believed that patients harboring staghorn calculi should not be treated (Segura, 1997). However, a better understanding of the natural history of staghorn stones has evolved. It is now generally accepted that, if left untreated, a staghorn calculus is associated with progressive deterioration of renal function. Additionally, morbidities associated with an untreated staghorn stone include pain, recurrent urinary tract infection, and sepsis events. Furthermore, patients with untreated staghorn calculi face an increased risk of death. Thus, untreated struvite staghorn calculi eventually destroy the kidney and pose a significant risk to the patient’s life. The American Urological Association (AUA) Nephrolithiasis Clinical Guidelines, released in 2005, recommended that in otherwise healthy individuals, newly diagnosed struvite staghorn calculi should be treated surgically (Preminger et al, 2005). Moreover, struvite stones must be removed completely to minimize the risk of continued urea-splitting bacteriuria. In examining the efficacy of SWL in the treatment of patients with renal calculi, passage of stone debris rather than fragmentation of the stone is the primary limiting factor (Renner et al, 1999). There is general agreement that stone free is the most rigorous definition of successful outcome of any stone removal procedure and that complete stone clearance should be the preferred goal of any intervention (Psihramis et al, 1992). However, because SWL outcome is dependent on spontaneous stone clearance, treatment results are often reported in terms of “success rates,” which may be defined as patients who are either stone free or who have asymptomatic, small, residual fragments. Various cutoff points between 2 and 5 mm are used in the literature to define the size of these fragments, making study comparisons difficult. In many cases, failure of SWL is not due to a failure of stone fragmentation but rather a failure to clear the resulting stone fragments. Failure to clear stone fragments is a concern, because it results in a higher re-treatment rate as well as a higher number of ancillary procedures. Clayman and associates (1989) suggested that in comparing the results of SWL and PNL or in comparing different lithotripters, the parameters of stone-free rate, re-treatment rate, and number of auxiliary procedures should be combined into an effectiveness quotient that may better express treatment results and allow one to compare different treatment modalities: For example, Netto and associates (1991), in a study comparing PNL and SWL for patients with lower pole calculi, reported overall stone-free rates of 93.6% and 79.2% for PNL and SWL, respectively; these values were not significantly different. However, the effectiveness quotients of 93.7% and 55.9% for PNL and SWL did differ significantly because this calculation incorporated the 41% re-treatment rate for the SWL group. The negative effect of an increasing stone burden (size and number) on the results of SWL has been described by a number of groups, dating from the initial reports of SWL to the present generation of lithotripters (Drach et al, 1986; Lingeman et al, 1986a; El-Assmy et al, 2006a; Tan et al, 2006). A now-axiomatic principle of SWL is that as stone burden increases, the stone-free rate declines and the need for ancillary procedures and re-treatment rises. Importantly, stone burden is not defined solely on the basis of the largest stone present in the kidney but also it takes into account the overall number of stones present. Furthermore, larger stone burdens are associated with a higher rate of residual stones, a point of particular concern in the treatment of patients with struvite calculi (Preminger et al, 2005). Figure 48–3 illustrates the effect of the size of solitary renal stones on the results of SWL. PNL, although more invasive and often associated with higher morbidity, achieves better stone-free rates than does SWL and is not affected by stone size (Lingeman et al, 1987a). Ureteroscopy, an alternative treatment for patients with renal calculi, is also negatively affected by increasing stone burden, although to a lesser degree than is SWL, because stone fragments are often removed or vaporized. Thus, as stone burden increases, PNL becomes more efficient than either SWL or ureteroscopy. Importantly, 50% to 60% of all solitary renal calculi are less than 10 mm in diameter (Cass, 1995; Renner and Rassweiler, 1999; Logarakis et al, 2000). Treatment results of SWL for this substantial group of patients are generally satisfactory and independent of stone location or composition. Although better results can be achieved with PNL or ureteroscopy for patients with stones smaller than 10 mm, these procedures are more invasive, are associated with greater morbidity, and may be reserved for special circumstances (e.g., anatomic malformation causing obstruction, SWL failure). Patients with calculi between 10 and 20 mm are often treated with SWL as first-line management. However, stone location and composition can meaningfully affect the results of SWL for patients with calculi in this size range and should be carefully considered. For example, SWL results for patients with 10- to 20-mm stones in the lower pole are inferior (55%) to SWL results for patients with stones in the upper and middle pole calyces (71.8% and 76.5%, respectively) (Saw and Lingeman, 1999). A prospective, randomized controlled trial compared SWL and PNL for patients with lower pole renal calculi; the stone-free rate for PNL was 95%, versus 37% for SWL (Albala et al, 2001). Stone composition merits consideration when evaluating treatment alternatives for patients with stones larger than 10 mm, as cystine calculi and brushite calculi both respond poorly to SWL treatment. This effect is particularly pronounced for stones larger than 15 to 20 mm. Therefore, patients with renal stones of 10 to 20 mm and factors predicting poor treatment outcomes with SWL should be advised about alternative therapeutic modalities. Both PNL and ureteroscopy are less affected by stone location and composition, and good results may be attained with these modalities for patients with 10- to 20-mm renal stones. Patients with renal calculi greater than 2 cm who are treated with SWL monotherapy commonly experience poor treatment outcomes, a fact that was first recognized over two decades ago in an NIH Consensus Conference. Interestingly, the 2 cm threshold for SWL first noted in that conference document is still valid in the present day (Consensus Conference, 1988). Murray and coworkers (1995) reported that SWL monotherapy for renal calculi greater than 3 cm yielded an overall success rate of 27% at 3 months follow-up. The best stone-free rate (60%) was obtained for stones smaller than 500 mm2 that were located primarily within the renal pelvis; the stone-free rate for stones with surface areas larger than 1000 mm2 was a dismal 8%. Notably, steinstrasse occurred in 23% of patients. El-Assmy and colleagues (2006a) reported on patients with large-volume renal calculi treated with SWL monotherapy. Long-term follow-up demonstrated a stone-free rate of 59%; significant complications occurred in 13%, and unplanned secondary procedures were required in 18.4% of cases. As an alternative to SWL for large-volume calculi, ureteroscopy emerged in the 1990s as a viable treatment option. Grasso and associates (1998) provided one of the earliest series of patients with large (>2 cm) upper urinary tract stones treated by ureteroscopy. One third of patients with renal stones required a second-look endoscopy; and in three patients with renal calculi, conversion to PNL was necessary. The overall success rate, defined as pulverization of the stone to dust or fragments smaller than 2 mm, after the second ureteroscopy procedure was 91%, which is comparable to PNL results. However, the 6-month follow-up data, which were available for 25 patients, demonstrated that only 60% of patients were stone free, whereas 24% had small lower pole debris and 16% had new stone growth. As surgical techniques and technology have evolved, ureteroscopy has been applied to patients with progressively larger stone burdens with acceptable results and morbidity (Mariani, 2007; Ricchiuti et al, 2007; Breda et al, 2008). In general, these treatment approaches have relied on a staged approach to achieve a successful outcome. Patients suffering from staghorn calculi remain a challenging problem for the practicing urologist. Most staghorn stones are composed of struvite, and factors that predispose to urinary tract infection and retained urine increase the likelihood of struvite stone formation (Gettman and Segura, 1999). However, other crystals, including cystine, calcium oxalate monohydrate, and uric acid, can assume a staghorn configuration. The conservative treatment of patients with staghorn calculi exposes the patient to an increased risk of renal loss as well as a mortality rate of up to 30% (Blandy and Singh, 1976; Rous and Turner, 1977; Koga et al, 1991). Therefore the ideal management of patients with staghorn calculi is composed of three stages. First, complete surgical removal of the entire stone burden is essential. If all of the infected stone debris is not evacuated, urea-splitting bacteriuria may persist, which can ultimately lead to eventual stone regrowth. The urologist should select the procedure or combination of procedures most likely to render the patient free of stone material while minimizing the risk of morbidity and mortality. Second, any metabolic abnormalities must be identified and appropriately treated. It has been reported that metabolic abnormalities are not uncommon in patients with infected stones (Segura et al, 1981). However, others have found that stone recurrence after complete elimination of calculi is uncommon (Silverman and Stamey, 1983). Different definitions of the term infection stone, and in particular the inclusion of mixed struvite and calcium oxalate stones in studies, probably explain these contradictory reports. In a group of patients with infection stones, those with pure struvite stones were significantly less likely to have metabolic abnormalities than were patients who had stones composed of a mixture of struvite and calcium oxalate (Lingeman, 1995; Lingeman et al, 1995c). Finally, anatomic abnormalities that may contribute to stasis within the urinary tract should be addressed. The morphologic classification into partial and complete staghorn calculi is inadequate, as demonstrated by Lam and colleagues (1992c), who reported considerable overlap in stone burdens of calculi grouped as partial or complete staghorn stones. When stone burden was assessed by stone surface area as measured on a kidney-ureter-bladder (KUB) radiographic image, for stones between 501 and 1500 mm2 an overlap between partial and complete staghorn calculi commonly occurs. To remedy this limitation several groups have proposed new classification schemes to better define staghorn calculi (Rocco et al, 1984; Griffith and Valiquette, 1987; Ackermann et al, 1989; Di Silverio et al, 1990). However, the cumbersome and subjective nature of these classification approaches has resulted in limited clinical use. Therefore, at present, the most accurate method to estimate the volume of a staghorn calculus is CT with three-dimensional reconstruction. This technique permits highly accurate determination of stone volume as well as the three linear dimensions of renal calculi and correlates well with the actual volume of the stone, as measured by water displacement (Lam et al, 1992c). Thiruchelvam and coworkers (2005) reported that an added benefit of three-dimensional image reconstruction is in planning for subsequent percutaneous stone removal. However, three-dimensional CT reconstructions are costly, time consuming, and not widely available, thus limiting the utility of this technology. Nadler and associates (2004) have reported the use of coronal reconstructions of axial CT images to calculate the craniocaudal length of stones, which can facilitate more economical stone volume calculations. Historically, patients with staghorn calculi were subjected to open surgical stone removal procedures. Overall, the stone-free rate after open surgery for patients with struvite staghorn stones was reported to be about 85%, with a 30% stone recurrence rate (Griffith et al, 1978). With the rise of endourology, however, minimally invasive procedures proved competitive with open surgery. In a comparison of PNL and anatrophic nephrolithotomy, Lingeman and associates (1987a) demonstrated that the stone-free rates of the two interventions were similar. Additionally, the convalescence, hospital stay, and blood transfusions were less for PNL. More recently, Al-Kohlani (2005) performed a randomized controlled trial comparing PNL and open surgery. Similar to the previous work of Lingeman they found that the morbidity, hospital stay, and operating room time all favored PNL, and the patients treated with PNL all returned to work sooner. The use of multiple endourologic techniques for the treatment of patients with staghorn stones is referred to as combination therapy or “sandwich therapy.” The most frequently used multimodal regimen was described by Streem and colleagues (1997) and consisted of a primary percutaneous stone debulking followed by SWL of any inaccessible, residual infundibulocalyceal stone extensions or fragments. After SWL a secondary percutaneous procedure was performed. These various stages are usually separated by 1 or 2 days. Stone-free rates for combined therapy are similar to those obtained by PNL alone or by open surgery (Lam et al, 1992b). The management of patients with staghorn stones by a combined approach must be viewed as primarily percutaneous in nature, with SWL being used only as an adjunct to minimize the number of access points required. Improved PNL techniques, incorporating the increasing use of flexible nephroscopy and providing complete or nearly complete clearance of stone material at the time of the primary procedure, may have decreased or eliminated the need for additional SWL treatment (Preminger et al, 2005). The 2005 AUA Nephrolithiasis Committee has published recommendations for the management of patients with staghorn calculi based on a meta-analysis of outcome data from published, peer-reviewed articles. According to the committee, all treatment options (SWL, PNL, combined PNL and SWL, open surgery) must be discussed with the patient (Preminger et al, 2005). As a guideline, however, PNL, followed by either SWL or repeated PNL, should be used for most patients with struvite staghorn calculi, with PNL being the initial element of the combination therapy. SWL and open surgery should not be used for most of these patients as a first-line treatment. PNL and SWL are equally effective in treating patients with small-volume staghorn stones when the renal anatomy is normal or nearly normal. Open surgery may be an option in unusual situations in which a staghorn stone is not expected to be removed by a reasonable number of PNL or SWL procedures. Nephrectomy is an option for the patient with a poorly functioning kidney harboring a staghorn stone. Although there are limited data for the treatment of pediatric patients with staghorn calculi, PNL is a safe and effective therapy. Pediatric patients often experience better stone-free rates than do adults with treatment by SWL. The stone-free rate with SWL monotherapy, reported by studies including only pediatric patients, is 78%; although more than one procedure is generally required to achieve this outcome; fortunately, complications are infrequent (Preminger et al, 2005). However, the developing kidney may be more susceptible to the bioeffects of SWL (Connors et al, 2006). Dretler (1988) first introduced the concept that stone fragility, defined as the readiness with which a stone is fragmented by SWL, is variable among stones of different composition. Saw and Lingeman (1999) subsequently reported that, when adjusted for size, cystine and brushite calculi are the most resistant to SWL, followed by calcium oxalate monohydrate; following, in descending order of resistance to fragmentation, are struvite, calcium oxalate dihydrate, and uric acid stones (Pittomvils et al, 1994; Saw and Lingeman, 1999). Stone composition affects not just resistance to fragmentation but also the type of fragments produced. Cystine and calcium oxalate monohydrate, in addition to being difficult to fragment, tend to produce relatively large pieces that may be difficult to clear from the collecting system (Pittomvils et al, 1994; Rutchik and Resnick, 1998). In general, patients with such stones (i.e., brushite, cystine, calcium oxalate monohydrate) should be treated by SWL only when the stone burden is small (i.e., <1.5 cm). Those patients with larger stones should preferentially be treated with PNL or ureteroscopy. Interestingly, the outcome of intracorporeal lithotripsy is also affected by stone composition. Teichman and colleagues (1998c) reported that the holmium laser was the most effective lithotrite for fragmenting struvite stones and the least effective lithotrite for fragmenting calcium oxalate monohydrate stones. These results are consistent with the known thermal threshold for each stone composition. Patients suffering from cystinuria present a unique challenge to the urologist. Assimos and associates (2002) reported that cystinuric patients have higher serum creatinine levels compared with a cohort of calcium oxalate stone formers. Importantly, they also reported that cystinuric patients are at greater risk for renal loss than are calcium oxalate stone formers and that open stone surgery for these patients is associated with higher serum creatinine concentration and potential renal loss. Furthermore, patients with cystinuria have been reported to be poorly compliant with medical therapy, increasing the likelihood of recurrent stone events (Pietrow et al, 2003a). Chow and Streem (1998) analyzed 31 cystinuric patients who underwent selected intervention for 61 stone events and reported that the probability of stone recurrence at 1 and 5 years was 27% and 73%, respectively. Achieving stone-free status prolonged the time to stone recurrence compared with patients left with residual fragments, a finding confirmed by Knoll and associates (1988). The high likelihood of repeated procedures underlines the need to select not just the least invasive treatment modality but also the most effective treatment modality. Treatment efficacy is of particular importance, as Barbey and associates (2000) reported that decreased renal function was more pronounced in cystinuric patients subjected to more stone removal procedures. Evan and associates (2006) have also reported that patients with cystinuria demonstrate anatomic findings, such as collecting tubule crystal plugging, as well as glomerular changes consistent with medical renal disease. These findings were more severe in subjects with extensive stone-forming histories, confirming the unique nature of this population. Therefore, when stone removal is required, a minimally invasive approach is preferred. SWL, when it is used unselectively to treat patients with cystine stones, yields poor results. Hockley and colleagues (1989) found that stone-free rates when applying SWL to calculi less than 20 mm or 20 mm or greater were 70.5% and 41%, respectively, whereas the stone-free rates for those who underwent PNL were 100% and 92%, respectively. Kachel and associates (1991) suggested the following treatment algorithm for patients with cystine stones: SWL monotherapy for cystine renal calculi 15 mm or smaller and PNL for stones larger than 15 mm in diameter. Rudnick and colleagues (1999) reported success with retrograde ureterorenoscopic fragmentation in patients with 1.5- to 3.0-cm renal calculi. This approach is especially appealing because it has an inherently low morbidity. Both Ahmed and associates (2008) and Trinchieri and associates (2007) have confirmed that endourologic approaches to patients with cystine calculi yield superior results, with minimal morbidity. Brushite calculi have a resistance to fragmentation that is surpassed only by that of cystine calculi (Dretler, 1988). Klee and associates (1991) described 30 patients with a total of 46 brushite stones. The overall success rate for patients treated by SWL monotherapy was 65% (with success defined as fragments < 4 mm), with a mean of 1.5 SWL sessions required per stone. However, only 11% of patients became stone free. PNL and ureteroscopy achieved 100% success rates and stone-free rates of 100% and 66%, respectively. Of 20 kidneys with residual fragments smaller than 4 mm, 12 had rapid regrowth to significant size within 3 to 12 months. Parks and associates (2004) found that SWL use was more frequent among brushite stone formers than among a similar cohort of calcium oxalate stone formers. Therefore, when brushite calculi are suspected or confirmed, a surgical treatment algorithm similar to that for cystine stones should be applied. Although resistance to SWL is generally common for very hard stones, it also characterizes the rare and very soft matrix calculi that are composed of as much as 65% organic matter (compared with 2% to 3% organic matter in most noninfected urinary calculi). Matrix stones are radiolucent and are often associated with urea-splitting bacteriuria. SWL is not an effective treatment modality for patients with these stones. These situations are usually best treated with PNL (O’Connor et al, 1990). SWL is usually ineffective because of the stone’s gelatinous nature, and ureteroscopy may be compromised by the large volume of stone material present (Bani-Hani et al, 2005). Another soft radiolucent stone is composed of indinavir, a protease inhibitor commonly used in the treatment of human immunodeficiency virus infection (Daudon et al, 1997). A significant number of patients who receive this drug develop symptoms or signs of indinavir nephrolithiasis (Saltel et al, 2000; Nadler et al, 2003). Reiter and coworkers (1999) reported an incidence of symptomatic stone episodes in 12.4% of 105 patients treated with indinavir. The mean time from the initiation of indinavir therapy until the acute stone episode was 21.5 weeks. Twelve of 16 stones were passed spontaneously. Kohan and colleagues (1999) described 13 symptomatic patients with indinavir stones; conservative therapy was successful in 11 patients, and 2 patients were treated by stent placement. Pure indinavir stones are not detectable with standard radiography or CT. However, some patients form indinavir stones that contain a calcium component, which may be radiographically visible (Sundaram and Saltzman, 1999). Hydration and analgesic therapy are recommended for the initial treatment of patients with indinavir stones. Indinavir therapy may need to be temporarily or permanently discontinued, in which case another protease inhibitor may be substituted. Invasive intervention may be necessary for patients with prolonged renal obstruction, signs of sepsis, or unremitting symptoms. The ability to predict stone composition and, consequently, the number of shockwaves required for complete stone fragmentation would be of great benefit in selecting appropriate treatment of patients with stone disease. If a stone is of a type not amenable to treatment by SWL, other modes of treatment could be pursued. However, except for cystinuric patients and patients who have had previous stone analysis, accurate prediction of stone composition based on imaging and the patient’s history is difficult. The ability of plain radiography to differentiate subtypes of calcium oxalate stones and possible relationships to stone fragility was first suggested by Dretler (Dretler, 1988; Dretler and Polykoff, 1996). A number of investigators subsequently examined the utility of x-ray patterns to predict stone fragility. In general, smooth edged stones with a uniform and homogenous structure require more shockwaves to fragment than do reticulated, spiculated stones with an irregular margin or structure. Additionally, stones that were more dense than bone (using either the 12th rib or a transverse process as a reference point) responded more poorly to SWL. Non–contrast-enhanced helical CT, which is presently the most commonly utilized method of evaluating patients with suspected renal colic, may be useful when trying to identify stone composition. Several in-vitro investigations of this technology have been reported. Mostafavi and associates (1998) first performed an in-vitro study that used the attenuation levels acquired by CT to accurately predict the chemical composition of pure urinary calculi. Similarly, Saw and associates (2000) found that, in an in-vitro investigation, CT was able to differentiate between stone groups (each containing at least 60% of one stone constituent) on the basis of absolute attenuation values. Joseph and associates (2002) have provided an in-vivo corollary when they reported that SWL success rates were significantly lower for those calculi with attenuation values greater than 1000 Hounsfield units (HU) than for those calculi with attenuation values less than 1000 HU. Gupta and associates (2005) have confirmed that there is a linear relationship between Hounsfield unit measurement of a stone and the likelihood of that stone fragmenting in a single SWL session. Other investigators have further advanced knowledge in this area, performing prospective studies to evaluate the utility of Hounsfield unit measurement in predicting stone fragmentation. Wang and associates (2005) confirmed that stone density greater than 900 HU (along with stone burden greater than 700 mm3 and irregular stone shape) is a predictor of a poor SWL outcome. El-Nahas and coworkers (2007), too, found that a stone density greater than 1000 HU predicted failure. Although CT attenuation values can distinguish some stone types in vivo, such as uric acid from calcium stones, the use of attenuation values alone results in considerable overlap; the range of values for calcium oxalate monohydrate and struvite stones does not allow these types to be confidently distinguished. Furthermore, it is not certain that the ease with which a stone is fragmented by SWL can be predicted by knowing only the major mineral composition of the stone. For example, cystine stones, which are considered difficult to break, have been shown in certain cases to break easily (Bhatta et al, 1989). Williams and associates (2003), too, reported that the variability in stone fragility to shockwaves is large, even within groups defined by mineral composition. It is likely that this variability in fragility could be due to variation in stone composition or structure, including variable amounts of secondary mineral in the stone, variation in the spatial arrangement of the secondary mineral within the stone, and variation in the layer structures of the primary and secondary minerals within a stone. Williams and associates (2002) also reported that displaying the data acquired by helical CT with use of bone windows can reveal remarkable internal structural detail of kidney calculi (Fig. 48–4 on the Expert Consult website Dual-source CT is a recent innovation in CT technology; it incorporates two unique x-ray sources, rather than a single x-ray source as in conventional CT. In addition to improved temporal resolution, dual-source CT scanners have the ability to operate the two x-ray sources simultaneously, which has the potential to differentiate materials on the basis of their unique energy-dependent profiles. Dual-source CT technology has been reported to reliably differentiate calcium stones from uric acid stones (Flohr et al, 2006) as well as calcium phosphate stones from calcium oxalate stones (Matlaga et al, 2008; Boll et al, 2009). UPJ obstruction in adults is commonly associated with urinary calculi. Furthermore, a stone at the UPJ can exacerbate the degree of preexisting obstruction and further compromise the renal unit (Rutchik and Resnick, 1998). The role of anatomic obstruction and associated urinary stasis in stone formation is not clearly established. Husmann and colleagues (1995) reviewed the records of patients with simultaneous UPJ obstruction and renal calculi and found that 71% of patients with nonstruvite stones had significant metabolic abnormalities. Matin and Streem (2000) have also reported that patients with UPJ obstruction are at increased risk for lithogenic factors associated with metabolic stone disease. Thus, in addition to anatomic obstruction, underlying metabolic abnormalities are commonly present in patients with UPJ obstruction. Although patients with stones and concomitant UPJ obstruction have traditionally been treated by open pyeloplasty and stone extraction, PNL with concomitant endopyelotomy can achieve good results with less morbidity. Endopyelotomy, defined as incision of the UPJ obstruction intraluminally, has an overall success rate between 67% and 88% (Ramsay et al, 1984; Van Cangh et al, 1989, 1994; Motola et al, 1993; Kletscher et al, 1995; Nadler et al, 1996; Albani et al, 2004; Knudsen et al, 2004). Percutaneous endopyelotomy can be combined with PNL, which permits efficient stone removal as well as careful endoscopic inspection of the UPJ. Success rates for combined PNL and endopyelotomy are comparable to endopyelotomy alone; a large, long-term review of endopyelotomy from the Mayo Clinic found that the presence of a stone did not adversely affect treatment outcome (Dimarco et al, 2006). Although retrograde endopyelotomy is another technique for the treatment of UPJ obstruction, the antegrade approach is preferable when renal calculi are present because it simplifies the stone removal aspect of the procedure. Laparoscopic pyeloplasty has emerged as a commonly applied treatment for patients requiring surgical repair of UPJ obstruction. Because stones are commonly present in such situations there has been interest in laparoscopic stone removal at the time of laparoscopic pyeloplasty. Ramakumar and colleagues (2002) initially reported the feasibility of laparoscopic pyeloplasty with concomitant pyelolithotomy. Ball and associates (2004) subsequently noted, however, that laparoscopic pyeloplasty with concomitant pyelolithotomy is most efficacious when it is applied to patients with limited stone burdens. As surgical instrumentation had advanced, Stein and associates (2008) have subsequently reported that increasing stone burdens can be efficiently treated, using laparoscopic, rather than endoscopic, instrumentation. Atug and associates (2005) have also reported the application of robotic technology, specifically robotic graspers, to accomplish pyelolithotomy at the time of a robotic pyeloplasty; this technique, too, permits the efficient removal of a stone burden. Calyceal diverticula are congenitally derived, nonsecretory, urothelium-lined eventrations of the renal collecting system that are filled with urine. A narrow neck communicating with the collecting system is typically present, which permits the diverticulum to fill passively with urine. Calyceal diverticula are uncommon, having been reported as incidental findings in 0.2% to 0.6% of individuals undergoing renal imaging (Middleton and Pfister, 1974; Timmons et al, 1975; Wulfsohn, 1980; Michel et al, 1985). Stones have been reported to form in 9.5% to 50% of these cavities and can cause pain and hematuria or harbor bacteria (Yow and Bunts, 1955; Williams et al, 1969; Middleton and Pfister, 1974). The role of metabolic factors versus urinary stasis in the pathogenesis of stone formation in calyceal diverticula is controversial. Burns and coworkers (1984) suggested that particle retention time, especially in the setting of a diverticulum, could be the cause of stone formation. However, several studies examining metabolic data have drawn conflicting conclusions. Although Hsu and Streem (1998) have reported metabolic abnormalities in 50% of 14 patients with calyceal diverticular calculi, Liatsikos and associates (2000) found a low incidence of metabolic abnormalities in 49 patients with calyceal diverticular stones. Matlaga and associates (2007) reported that it is likely a combination of both urinary stasis and metabolic factors that incite stone formation in these structures.
Historical Overview
Kidney Calculi
Ureteral Calculi
The Rise of Endourology
Ureteroscopy
Percutaneous Stone Removal
Extracorporeal Shockwave Lithotripsy
Renal Calculi
STONE FACTORS
RENAL ANATOMIC FACTORS
CLINICAL (PATIENT) FACTORS
Preoperative Evaluation
Natural History
Calyceal Stones
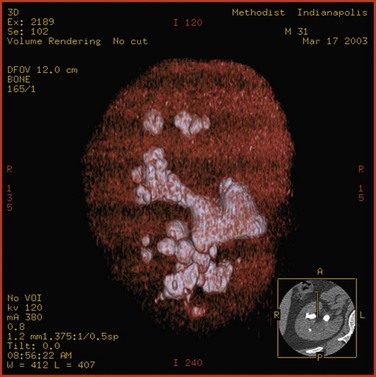
Staghorn Calculi
Stone Factors
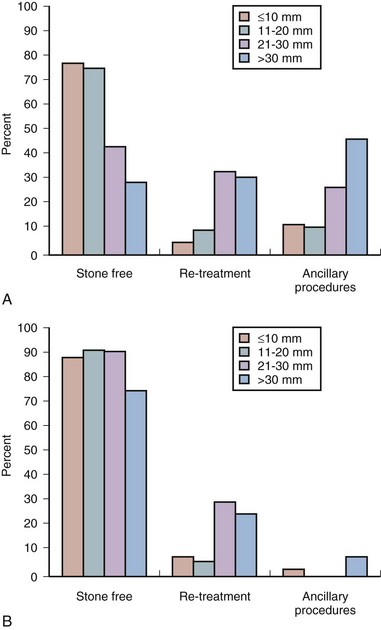
Treatment Decisions by Stone Burden
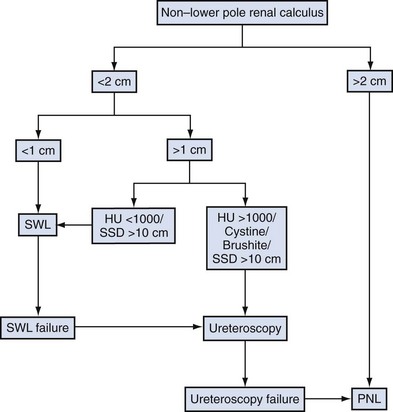
Nonstaghorn Calculi
Staghorn Calculi
Classification of Staghorn Calculi
Surgical Management of Staghorn Calculi
Treatment Decisions by Stone Composition
![]() ). It is clear that CT provides a wealth of information about stone characteristics. However, additional work is needed to determine the utility of this powerful imaging tool in determining the susceptibility of a given stone to SWL.
). It is clear that CT provides a wealth of information about stone characteristics. However, additional work is needed to determine the utility of this powerful imaging tool in determining the susceptibility of a given stone to SWL.
Renal Anatomic Factors
Ureteropelvic Junction Obstruction
Calyceal Diverticula
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgical Management of Upper Urinary Tract Calculi