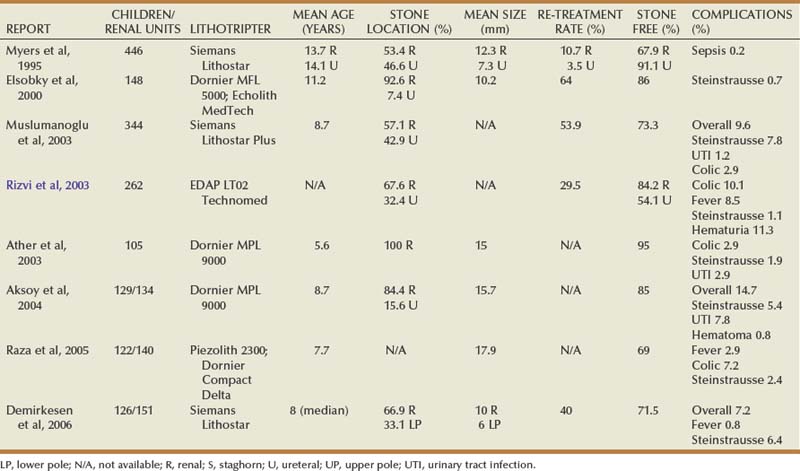
Michael C. Ost, MD, Francis X. Schneck, MD Prior to 1990, nephrolithiasis was responsible for 1 in 1000 to 1 in 7600 hospital admissions annually throughout the United States (Nimkin et al, 1992). In recent years, however, a dramatic increase in pediatric urolithiasis has been observed (Srivastava and Alon, 2005). The authors would concur with this observation, especially among adolescents without known metabolic disturbances. It has been speculated that diets rich in sodium and carbohydrates may be contributing to the etiology of urolithiasis in this cohort of children. Pediatric stone disease has always been more prevalent in underdeveloped countries. Of concern is the observation that pediatric stone disease is becoming more prevalent in the western hemisphere. Many pediatric patients with urolithiasis have metabolic abnormalities (Jayanthi et al, 1999). Therefore there has been an increased demand on pediatric urologists to manage both simple and complex urolithiasis in all pediatric age groups. Often, a complete metabolic blood profile and a 24-hour urine collection are obtained once a stone is confirmed by imaging. Unfortunately, there are no accepted standard reference ranges for 24-hour urine analyses in the stone-forming pediatric population. Indeed, many pediatric urologists use adult standards to direct medical treatment, because specific reference ranges are not readily available or require additional complicated calculations. Regrettably, there are significant differences between the normal ranges of urine chemistries in children and adults (Battino et al, 2002; DeFoor et al, 2005a). In addition, conflicting reports exist as to the commonality of metabolic disturbances in the presence of calcium-based stones (Sternberg et al, 2005). In the preliminary evaluation of pediatric stone disease, it is common to obtain 24-hour urine values for creatinine, sodium, calcium, oxalate, uric acid, and citrate. The very cumbersome nature of a 24-hour urine collection often limits its accuracy in the pediatric population. In light of this random urine spot sampling, ratios have been used. Urine calcium to urine creatine ratios (Uca : Ucr), for example, have been used with sensitivities and specificities up to 90% and 84%, respectively, in the evaluation of hypercalciuria, a known risk factor for urolithiasis (Mir and Serdaroglu, 2005). However, such a test is limited to one urinary metabolite and cannot be relied upon to monitor responses to various medical treatments. Furthermore, it is unreasonable to rely solely on urinary calcium excretion values as an indication of overall metabolic disturbances. It has been suggested that measurement of urinary supersaturation products (calcium oxalate, urate) may help to improve identifying those children at risk for stone formation. DeFoor and colleagues (2006), for example, most recently determined that supersaturation levels of calcium oxalate, as well as calcium to creatinine levels, were significantly higher in children with stones compared with controls. This difference, however, may have been reflective of differences found in urinary volumes. Lande and colleagues (2005) have demonstrated that low urine volumes in children often negate the benefit of pursuing urine supersaturation products in a stone workup. Such conflicting data often confuses clinicians as to what urinary evaluation to pursue in the workup and management of pediatric stone disease. Stone disease in the pediatric population has genetic, anatomic, metabolic, and dietary causes. There are numerous genetic causes of hypercalciuric nephrolithiasis alone that contribute to pediatric stone disease (Stechman et al, 2009). In this regard, the medical treatment always accompanies the endourologic management. To fully treat the pediatric stone patient, there must be a focus on prevention through diet and medication monitoring. For this reason, the pediatric nephrologist is a critical player in the management and surveillance of these children. Details regarding the metabolic workup and medical treatment of pediatric stone disease are beyond the scope of this chapter. Numerous pediatric nephrology resources are available. Alon (2009), for example, provides an excellent pediatric nephrology review, including medication dosing by weight. The website Litholink (www.litholink.com) can provide valuable information to parents and pediatric urologists alike. Radiographic imaging in children is similar to imaging in the adult patient. The workup begins when the diagnosis of a calculus is suspected, and the radiographic study is determined by factors such as acuity of presentation and the practices of the treating hospital. Many, if not most, children with renal colic present to an emergency room, and unenhanced helical computed tomography (CT) has become the most accurate and efficient first choice in initial imaging. Typically, thin-slice helical protocol is defined by narrow collimation (≤5 mm). CT is not only able to visualize the entire urinary tract for the tiniest calculus, but it has the potential of ruling out alternative diagnosis. Unenhanced helical CT of the abdomen and pelvis to evaluate urinary tract calculi was first described in 1995 and is the mainstay for calculus imaging in adolescents and adults, replacing more traditional studies, such as intravenous pyelography (IVP), a kidney-ureter-bladder (KUB) film, and ultrasonography (Smith et al, 1995). With increased usage and familiarity with the technique, its application has expanded into the pediatric population with similar advantages. These advantages include high sensitivity and specificity, ready availability and speed in assessment, and intravenous (IV) contrast is not necessary. Because children are at significant lifetime risk for recurrence of urolithiasis, judicious use is necessary to limit radiation exposure, particularly gonadal exposure, and concerns of potential increase risk of future malignancy. CT is highly accurate in stone assessment, exceeding 96% sensitivity and specificity independent of the location of the calculus (Smith et al, 1996b; Hamm et al, 2001; Heneghan et al, 2003; Palmer et al, 2005). CT is also helpful in demonstrating secondary signs of acute obstruction, such as hydroureteronephrosis, renal enlargement, and perinephric or periureteric stranding; however, the latter findings may be less obvious or absent in pediatric patients due to comparably less retroperitoneal fat (Smith et al, 1996a; Smergel et al, 2001; Strouse et al, 2002). Progress has been made in developing unenhanced CT protocols that minimize the radiation dosage while not compromising diagnostic information (Heneghan et al, 2003; Cody et al, 2004; Singh et al, 2009). Experimental protocols using anthropomorphic pediatric phantoms have been used in a number of studies to determine specific organ doses, calculate effective dose, determine the lifetime attributable risk (LAR) for cancer incidence, and relative risk of cancer induction from a single scan under both standard- and low-dose modes (Brisse et al, 2009; Kim et al, 2010). Computer-simulated dose reduction has been useful in determining diagnostic thresholds in children. Compared with standard protocols, halving the dose to 40 mA for children weighing 50 kg or less does not significantly affect the diagnosis of pediatric renal stones (Karmazyn et al, 2009). Spielmann and colleagues (2002) found excellent detectability of calculi measuring 2 to 8 mm using much lower amperage, with an almost threefold decreased estimated radiation dose compared with standard protocols. Perhaps most importantly, radiation exposure can also be reduced effectively by reducing the number of CT examinations performed for poor clinical indications, scanning only the anatomic region of interest, and not performing either unenhanced and contrast-enhanced scanning unless absolutely necessary (Cohen, 2009). Ultrasonography has a more limited role in the assessment of urolithiasis compared with CT but has the distinct advantage of no associated ionizing radiation. Therefore ultrasonography should be considered as a screening tool in the workup for nonemergent abdominal or flank pain. Ultrasonography, although useful in the evaluation of renal calculi or hydronephrosis, is technically limited for use in diagnosing a ureteral stone, with the possible exception of the very distal ureter or bladder. Especially in the acute setting, that is, symptomatic presentation with renal colic or hematuria, ultrasonography is less useful in detecting or directing management of urolithiasis. Palmer and colleagues (2005) demonstrated that ultrasonography was nondiagnostic in 41% of children compared with 5% using CT, and it failed to detect ureteral calculi in 62% of pediatric patients. Similar results have been reported in adults, confirming that ultrasonography is of limited value in the workup of urolithiasis (Fowler et al, 2002). Symptomatic children with a known history of renal urolithiasis, or children on observation protocols, may suffice with ultrasonography (US) and KUB alone, thereby limiting exposure to ionizing radiation. Additional support favoring US versus CT in the diagnosis of pediatric nephrolithiasis is also rooted in the ultimate clinical decision about how the child is to be treated. In a prospective study comparing CT and US, Passerotti and colleagues (2009) found that although CT was a more sensitive study in detecting stones in children, the difference in usefulness between the two radiologic techniques was not clinically significant. Conservative management of pediatric nephrolithiasis is considered first-line treatment, provided there is no evidence an obstructing stone is harboring an infection or a child is failing to thrive as the result of his or her stone disease. Clinical scenarios, including fever, anorexia greater than 24 hours, persistent nausea and vomiting, and/or pain refractory to conservative measures prompts endourologic intervention. In the instance of a stone in a solitary kidney, early intervention versus conservative treatment is favored. In managing stone disease in the pediatric population, it is important to note that renal calculi less than 3 mm are likely to spontaneously pass, and stones greater than or equal to 4 mm in the distal ureter are likely to require endourologic treatment (Van Savage et al, 2000). This information should be relayed to caregivers and parents. If a ureteral stent is placed acutely in children for the clinical circumstances described above, definitive endourologic therapy is delayed 2 to 4 weeks to allow for decompression, ureteric orifice dilation, resolution of edema, and proper treatment and clearance of any infection if need be. There have been many studies in adults evaluating the efficacy of medical expulsive therapy to facilitate distal stone passage. Use of α antagonists, calcium channel blockers, and steroids has been shown to be effective. Based on efficacy demonstrated in the adult population (Porpiglia et al, 2004), α-receptor antagonists, such as tamsulosin, may be offered on an individualized basis as adjunctive therapy to facilitate ureteral expulsion in children. To date, however, there is a lack of published data capturing the pediatric population that prove the superiority of these agents versus standard pain medication. A Turkish study, for example, demonstrated that daily administration of 0.03 mg/kg of the α antagonist doxazosin versus an analgesic alone did not show superior expulsive results regarding distal ureteral stones up to 10 mm in children 2 to 14 years of age (Aydogdu et al, 2009). Special considerations in the endourologic management of stone disease in children include preservation of renal development and function, prevention of radiation exposure, and minimizing the need for re-treatment. Despite advances in endourologic equipment and technique, controversy remains regarding the contribution of SWL to future development of diabetes or hypertension, and whether ureteric orifice dilation during ureteroscopy (URS) leads to ureteral stricture formation or development of vesicoureteral reflux. International consensus is lacking as to the most effective surgical management of pediatric stone disease due to lack of prospective randomized trials comparing treatment modalities and disparity in the access to emerging technologies. Regardless of treatment modality, the presence of residual stone fragments is associated with adverse clinical outcomes (Afshar et al, 2004), and every attempt should be made to achieve a stone-free status. The surgeon’s experience is paramount to facilitate complete stone clearance and minimize re-treatment rates. The decision regarding the most efficacious primary treatment modality must be individualized per child based on age, anatomy, location, and composition of the stone burden. In line with the 2008 American Urologic Association’s best practice statement on antibiotic prophylaxis, less than or equal to 24 hours of perioperative antibiotics are indicated in all patients undergoing upper tract instrumentation (Wolf et al, 2008). In children, appropriate agents include trimethoprim-sulfamethazole, first- and second-generation cephalosporins, and ampicillin in combination with an aminoglycoside. A urine culture is mandatory before all upper tract procedures to determine if the urine is sterile, and culture results are used to guide preoperative antibiotic therapy, particularly for percutaneous procedures, patients with high-grade obstruction, or patients with an indwelling stent (Wu and Docimo, 2004). In the authors’ practice, children with a negative urine culture undergoing uncomplicated URS procedures receive perioperative cefazolin, and all children undergoing a percutaneous procedure or who have a preexisting ureteral stent/nephrostomy tube receive a fluoroquinolone or ampicillin/gentamicin. Use of postoperative antibiotics is controversial and is determined on an individual basis, especially with recent data demonstrating an increased risk of developing resistant bacterial strains with prolonged use of antibiotic prophylactic therapy (Conway et al, 2007). The emergence of shock wave lithotripsy (SWL) revolutionized the minimally invasive treatment of adult urolithiasis during the early 1980s. Since the initial report on successful SWL use in children in 1986 (Newman et al, 1986), large series have reported complication, safety, and stone-free rates comparable to adult cohorts (Table 135–1) (Myers et al, 1995; Elsobky et al, 2000; Ather and Noor, 2003; Muslumanoglu et al, 2003; Rizvi et al, 2003; Aksoy et al, 2004; Raza et al, 2005; Demirkesen et al, 2006). When used as a primary treatment option for upper tract calculi, SWL efficacy ranges from 68% to 84% (Rizvi et al, 2003; Myers et al, 1995; DeFoor et al, 2005b). SWL has been a preferred treatment modality for uncomplicated renal and proximal calculi less than or equal to 15 mm in the pediatric population. In a contemporary series of 216 children (mean age 6.6 years) with a mean stone size of 14.9 mm and who were undergoing SWL with the Dornier HM3 lithotripter, Landau and colleagues (2009) reported a 3-month stone-free rate of 80%, demonstrating that efficacious stone-free rates can be achieved in appropriate candidates. Complications rates are minimal and range in severity from hematuria and ecchymosis to obstruction with sepsis (Farhat and Kropp, 2007). Although well tolerated in children, current stone-free rates with SWL are difficult to interpret from the existing body of data due to discrepancies between studies regarding type of lithotriptor, number of shocks administered, and re-treatment rates. Recent data suggest that stone-free rates in children with a history of urologic condition or urinary tract reconstruction are quite low (12.5%), and, with alternative surgical techniques available, children may be better served with URS or PCNL (Nelson et al, 2008). Despite encouraging results, SWL has not been approved by the Food and Drug Administration for use in children, although it is a widely accepted treatment modality. General anesthesia is administered in a majority of smaller children to avoid both patient and stone motion and the need for repeated repositioning. With modern lithotriptors, intravenous sedation has been successfully employed in select older children (Aldridge et al, 2006). Bowel preparation is seldom used in order to avoid dehydration and electrolyte imbalance postoperatively. The number of shocks delivered and the kilovoltage used vary per lithotripter, but the current consensus is that low-power settings (17 to 22 kV) should be used to prevent stone migration during the procedure, with 3000 shock waves per session (<2000 in very young children) (Farhat and Kropp, 2007). A recent report assessed and compared the number and intensity of shock waves required for stone fragmentation in 44 children (mean age 5.9 years) and 562 adults (mean age 40.9 years). With an equivalent number of sessions (1.1 vs. 1.1), the mean number of shock waves (950 vs. 1262, P < .001) and the kV required (11.8 vs. 12.4, P < .001) were significantly reduced in the pediatric cohort (Kurien et al, 2009). Although early series focused primarily on the feasibility, safety, and efficacy of SWL in children, recent efforts have centered on identifying demographic, anatomic, and stone-related prognostic factors for treatment success. SWL is currently considered the primary treatment for upper tract calculi less than or equal to 15 mm in children (Farhat and Kropp, 2007), but evidence supporting this stone size cutoff is lacking. Ather and Noor (2003), for example, analyzed the correlation between stone size and clearance in 105 children younger than 14 years. They reported an overall stone-free rate of 95% after a mean of 1.7 SWL treatments, with 5% of patients requiring additional procedures as adjuncts to SWL. In this cohort, mean stone size in the treatment success group was 14 mm compared with 16 mm in the treatment failure group. By contrast, Elsobky and colleagues (2000) reported a 91% stone-free rate versus 75% stone-free rate for a mean stone diameter less than 10 mm and greater than 10 mm, respectively. Recently, Shouman and colleagues (2009) reported on a series of 24 children with a mean stone size of 31 mm undergoing SWL with the Dornier DoLi S device. In 53 sessions requiring a mean number of 3489 shock waves per session, stone-free and complication rates were 83.3% and 25%, respectively. Although it is possible to treat very large stone burdens with SWL, concerns include the necessity of more shock treatments, more frequent re-treatment sessions, and increased risk of postoperative obstruction. Further study delineating a clear size cutoff for uncomplicated upper tract stone burden is required to effectively counsel parents regarding the most effective first-line therapy for renal calculi between 1 and 1.5 cm. Renal anatomy and stone location has been the subject of recent interest. The subject of frequent debate in the adult population, the most effective management of lower pole calculi in children has yet to be determined. Stone-free rates from initial small retrospective SWL series range from 56% to 61% (Ozgur Tan et al, 2003; Onal et al, 2004) with re-treatment rates as high as 40% (Onal et al, 2004). SWL failure and re-treatment rates were associated with increased mean stone burden (Onal et al, 2004), increased infundibular length (Ozgur Tan et al, 2003), and an infundibulopelvic angle greater than 45 degrees (Ozgur Tan et al, 2003). Staghorn calculi are uncommon in children and represent a management challenge. Although monotherapy success rates are low in adults, acceptable stone-free rates in children have been achieved with SWL. In 23 children with a mean stone burden of 1.6 cm who were stratified by age, Lottmann and colleagues (2001) reported an overall stone-free rate of 82.6%, with only one case of symptomatic obstruction. A ureteral stent was placed in 22% of children, and these authors reported an 88% stone-free rate in children less than 2 years old compared with 71% in children 6 to 11 years old. In 42 children with a mean stone burden of 3.2 cm and stratified by ureteral stent placement, Al-Busaidy and colleagues (2003) reported an overall stone-free rate of 79%. Although stent placement did not affect stone-free rates, they found that stent placement significantly reduced the major complication rate. The superior success rates with SWL monotherapy in children compared with adults have been attributed to softer stone composition, smaller relative stone volume, increased ureteral compliance to accommodate stone fragments, and smaller body volume to facilitate shock transmission. SWL safety and efficacy have been demonstrated even in very young children. McLorie and colleagues (2003) treated 34 children younger than 3.5 years (mean age 23 months) and reported an 86% overall stone-free rate (66% after one treatment) without major complications. Treatment of proximal ureteral stones has achieved similar success rates compared with treatment of renal stones in most pediatric series, although ureteral stenting is more commonly employed to aid in stone localization and clearance (Myers et al, 1995). Treatment of mid- to distal ureteral calculi has historically been avoided in children due to difficulties with localization over the sacroiliac joint and concern regarding possible injury to developing reproductive systems. The greater and lesser sciatic foramen has been explored as a potential blast path to treat distal stones in children. SWL success by stone composition is similar between the adult and pediatric populations. Cystine stones are uniquely challenging due to their durability and high recurrence rates. Although SWL monotherapy has demonstrated variable results in adults, there are few reports in the pediatric population. In a small recent series, Slavkovic and colleagues (2002) reported a 50% stone-free rate in 6 children with a cystine stone burden ranging from 0.2 to 2.5 cm. Although stone-free rates were low, fragmentation was achieved in 100% of patients, and the stone dissolution was achieved with medical therapy in the remaining children following SWL. Some authors have proposed that cystine stones formed within 2 years of therapy may be more easily fragmented with SWL and that stone number, and not diameter, may be more predictive of success (Farhat and Kropp, 2007). In children, there is currently no consensus regarding the maximum size of residual stone fragments (RF) that are considered clinically significant, and as a result, there is no clear definition as to what constitutes “stone-free” status (Wu and Docimo, 2004; Farhat and Kropp, 2007). Although children have been shown to have a greater capacity to clear fragments than adults (Gofrit et al, 2001), the presence of RFs have been correlated with adverse clinical outcomes (Afshar et al, 2004). Afshar and colleagues (2004) followed 26 renal units with RFs less than or equal to 5 mm and reported that although 31% were asymptomatic with no fragment growth, 69% had adverse clinical outcomes, including RF growth or clinical symptoms. Patients with RF had a significant increase in adverse clinical outcome compared with stone-free subjects, and the presence of metabolic disorders was associated with RF growth (Afshar et al, 2004). For these reasons, metabolic evaluations are now routinely being performed in children with a history of calculi, and every attempt should be made to achieve stone-free status. Although SWL is well tolerated in children with few complications, stone-free rates following single-session monotherapy can remain as low as 44% (Muslumanoglu et al, 2003). As a result, children are subjected to multiple treatments requiring general anesthesia (Aldridge et al, 2006). The need for multiple treatment sessions is concerning, because the effects of shock waves on renal tissue are unclear. A growing body of evidence in adults indicates that shock waves result in renal vessel vasoconstriction and that renal tubular injury and subcapsular hematoma from cavitation and shear forces are dependent on the kilovoltage applied (Lingeman et al, 2003). In a large series of 340 adult patients with a mean follow-up of 19 years post-SWL, (Krambeck and colleagues (2006) reported an increased risk of hypertension and diabetes mellitus related to bilateral treatment, number of administered shocks, and treatment intensity. Although these results are concerning, differences between pediatric and adult populations and limitations inherent to a questionnaire-based retrospective study make application of these data in children difficult. Retrospective studies with limited follow-up in children have reported that SWL and PCNL do not cause renal morphologic or functional alteration as measured by glomerular flow rate (GFR) and serial dimercaptosuccinic acid (DMSA) functional studies (Wadhwa et al, 2007), but long-term data are unavailable to date. To eliminate confounding variables and fully address the risks of chronic renal damage from SWL, long-term prospective data in children are clearly required.
Evaluation
Metabolic Workup
Imaging
Conservative Management
Management of Upper Urinary Tract Calculi
Pediatric Considerations
Antibiotic Use
Shock Wave Lithotripsy
Shock Wave Lithotripsy Technique in Children
Stone Size, Location, Composition, and Patient Age
Limitations and Concerns
Surgical Management of Pediatric Stone Disease