7 Surgical Management of Apical Vaginal Wall Prolapse
7-1 Laparoscopic Trocar Placement Used for Reconstructive Surgical Procedures
7-2 Laparoscopic Adhesiolysis before Laparoscopic Colpopexy
7-3 Laparoscopic Sacral Colpopexy
7-4 Laparoscopic Colposuspension
7-5 Sacrospinous Colpopexy with Anterior Mesh Placement and Posterior Repair
7-6 Techniques to Test for Occult Stress Incontinence
7-7 Total Vaginal Mesh Procedure
7-8 High Uterosacral Vaginal Vault Suspension Performed after Vaginal Hysterectomy
7-9 High Uterosacral Vaginal Vault Suspension for Posthysterectomy Prolapse
7-10 High Intraperitoneal Vaginal Vault Suspension—Revisiting this Pelvic Anatomy
7-11 Laparoscopic Release of Vaginal Scarring before Laparoscopic Colpopexy
Background
In reviewing the literature, the abdominal sacral colpopexy (ASC) remains the “gold standard” in the management of vault prolapse. (Nygaard et al, 2004; Maher et al, 2007) The Cochrane review noted three randomized controlled trials (RCTs), in which the ASC was associated with a lower rate of recurrent vault prolapse and less dyspareunia, as compared with sacrospinous colpopexy. However, the abdominal approach was also associated with longer surgical time and admission time, greater blood loss, and a slower return to performing activities of daily living.
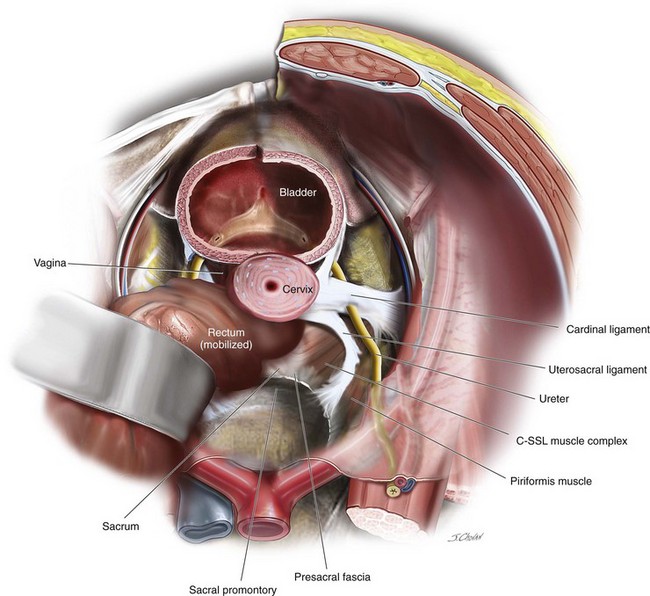
The uterosacral vault suspension remains a popular vaginal option. In a recent metaanalysis, Margulies and colleagues (2010) reviewed over 800 uterosacral cases and found the success rates for anterior, apical, and posterior compartments were 81%, 98%, and 87%, respectively. They also reported that associated preoperative stage III anterior compartment prolapse was associated with a significantly higher failure rate than stage II anterior compartment prolapse. Ureteric obstruction was the most common complication and was reported in 15 patients (1.8%). Figure 7-1 demonstrates the relationship among the uterosacral ligament, the ureter, the coccygeus–sacrospinous ligament (C-SSL) muscle complex, and the presacral fascia. In 10 patients, the removal of the offending uterosacral suspension suture relieved the obstruction, and ureteral re-implantation was required in 5 patients (0.6%). Blood transfusion was reported in 11 patients (1.3%). Intraoperative complications were low and included a cystotomy during hysterectomy (0.1%), and 2 patients had bowel injuries (0.2%). (Margulies, Rogers, Morgan, 2010) In a recent RCT, Rondini and associates compared open sacral colpopexy (n = 54) and high uterosacral ligament vault suspension (HUSLS) (n = 56) and demonstrated similar findings to the sacrospinous colpopexy previously reported. Higher objective success rates and lower rates of recurrences were reported in the anterior and posterior compartments. Although the reoperation rate for prolapse was significantly lower after sacral colpopexy (5% as compared with 17.8% in the uterosacral suspension group), both intraoperative complications (3.7% versus 0%, p = 0.15) and postoperative complications (20.4% versus 7.3%, p = 0.047) were higher after the sacral colpopexy, as compared with HUSLS.
After the uterosacral ligament suspension (USLS) and sacrospinous colpopexy, recurrent anterior compartment prolapse remains the most common site of failure. With this in mind, when vaginally performing apical prolapse surgery, some surgeons are now incorporating anterior compartment mesh with sacrospinous colpopexy and uterosacral suspensions; however, little data on the efficacy of this approach are available at this stage. Obliterative procedures are also an option in the older patient who is not sexually active. (See Chapter 10 for a detailed discussion of the procedures.)
In an attempt to decrease the perioperative morbidity associated with open sacral colpopexy, the laparoscopic sacral colpopexy (LSC) (Higgs, Chua, Smith, 2005; Paraiso et al, 2005; Klauschie et al, 2009; North et al, 2009), the robotic sacral colpopexy (Paraiso et al, 2011), and the vaginal mesh procedures (VMPs) (Fatton et al, 2007; Caquant et al, 2008) have increased in popularity. A recently completed RCT, in which the LSC and the total vaginal mesh (TVM) technique were compared with a 2-year follow-up, demonstrated a significantly higher anatomic success rate (LSC at 77% versus TVM at 43%), higher patient satisfaction (87 patients for LSC versus 79 patients for TVM,) longer total vaginal length (TVL) (LSC at 8.83 cm versus TVM at 7.81 cm), and significantly lower reoperation rate after the LSC, as compared with TVM (5% versus 22%, respectively). (Maher et al, 2011) The high reoperation rate after VMPs was also reported by Diwadkar and associates (2009) who systematically examined the complication and reoperation rates after traditional vaginal surgery, sacrocolpopexy, and vaginal mesh kit procedures. Complications were classified using the Clavien-Dindo grading system—grade IIIa (intervention not requiring general anesthesia) and grade IIIb (intervention under general anesthesia) rates were highest in the mesh kit group as a result of higher rates of mesh erosion (198 of 3425) and fistulae (8 of 3425). Reoperation rates for prolapse recurrence were highest in the traditional vaginal surgery group (308 of 7827). The total reoperation rate was greatest in the mesh kit group (291 of 3425, 8.5%). (Diwadkar et al, 2009)
The shorter TVL after the TVM procedure, as compared with LSC and reported in one prospective RCT (Maher et al, 2011), has also been reported in a multicenter retrospective chart review; this review compared Prolift VMP (206 patients), USLS (231 patients), and ASC (305 patients). No difference in apical success after VMP (98.8%) was demonstrated, compared with USLS (99.1%) or ASC (99.3%); however, the average elevation of the vaginal apex was lower after VMP (–6.9 cm) than USLS (–8.05 cm) and ASC (–8.5 cm) (both p = 0.001). (Sanses et al, 2009) In contrast, a recently prospective case series of 46 women with vault prolapse at 12 months reported excellent outcomes with a 91% overall objective success rate, a mesh erosion rate of 15% with the TVL decreasing from 8.8 to 8.5 cm postoperatively. (Milani, Withagen, Vierhout, 2009) Recently, Iglesia and colleagues (2010) reported a double blind RCT comparing Prolift (33 patients) and uterosacral native repair (32 patients) for vaginal prolapse. They found at 10 months the recurrence rate was 59% in the mesh group, as compared with 70% in the nonmesh group (p = 0.28). Unfortunately, the study was terminated early because a predetermined review board mesh erosion rate of 15% was surpassed. The authors questioned the value of polypropylene mesh grafts in prolapse surgery. More recently, Withagen and associates (2011) compared native tissue repairs in 97 women with Prolift mesh repairs in 93 women, all of whom underwent prior prolapse surgery. At 1 year, the authors reported the recurrence rate in the treated compartment was 45.2% in the conventional (nonmesh) group, as compared with 9.6% in the mesh group (p <0.001; odds ratio [OR], 7.7, 95% CI 3.3 to 18. No differences in subjective assessment and patient satisfaction were identified, and the mesh extrusion rate was 17%. On closer evaluation, this study failed to control selection and reporting bias and to report a meaningful definition of the objective assessment. Preoperatively, the nonmesh group is significantly different from the mesh group on a number of important preoperative assessments, and readers should be cautious when drawing conclusions from this trial. Unfortunately, 7 years after commercial mesh kits have been introduced into the market, no definitive level-one evidence confirms that these interventions are superior to the native tissue repairs in the management of apical prolapse.
In July 2011, the U.S. Food and Drug Administration (FDA) released a transvaginal mesh alert after an increased number of adverse events were reported; (the full document is available at http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm). Mesh erosions account for 38% of these alerts and occur in an estimated 10% of patients with over one half requiring at least one surgical intervention to address the erosion. Interestingly, vaginal pain and dyspareunia, together, account for the greatest number of reports (39%) and remain poorly characterized and reported in the literature. After conducting an extensive literature review, the FDA came to the following conclusions relevant to the apical compartment:
1. Mesh augmentation for transvaginal apical or posterior repair does not appear to provide any added benefit, compared with traditional native tissue suture repair.
2. Mesh abdominally placed for pelvic organ prolapse (POP) repair may result in less recurrent prolapse and, when compared with transvaginal meshes, appears to have lower complication rates.
The FDA also suggested that clinicians adhere to the following recommendations:
1. Recognize that, in most cases, POP can be successfully treated without mesh, thus avoiding the risk of mesh-related complications. Mesh surgery should only be performed after weighing the risks and benefits of surgery with mesh versus all surgical and nonsurgical alternatives.
2. The following factors should be considered before placing surgical mesh:
3. Inform the patient about the benefits and risks of nonsurgical options, nonmesh surgery, surgical mesh abdominally placed, and the likely success of these alternatives, compared with transvaginal surgery with mesh.
4. Notify the patient if mesh will be used in her POP surgery, and provide the patient with information about the specific product used.
5. Ensure that the patient understands the postoperative risks and complications of mesh surgery, as well as the limited long-term outcomes data.
• The safety of transvaginal meshes has not been established.
• Depending on the compartment, the efficacy of transvaginal meshes has not been established to be more effective than traditional repairs.
• Safety and efficacy of mesh at sacral colpopexy has been established.
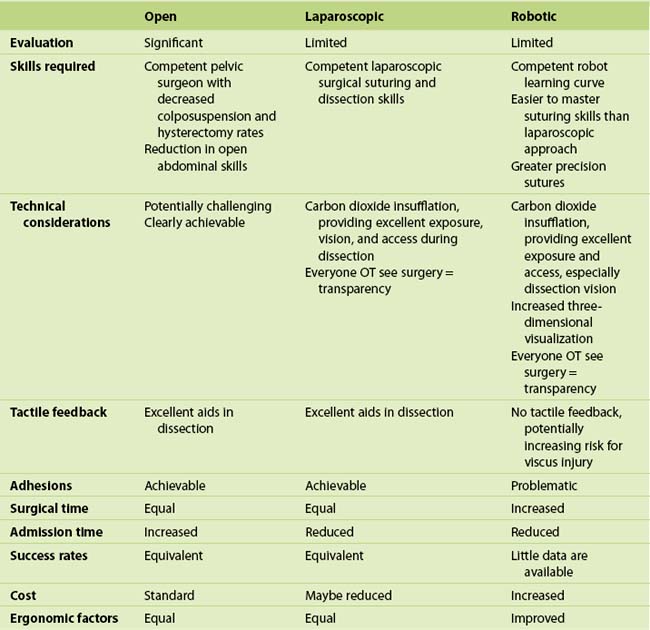
Currently, most ASCs can be endoscopically performed. The robotic approach to the sacral colpopexy is discussed fully in Chapter 6; however, in a recent RCT in which robotic and laparoscopic approaches were compared, Paraiso and colleagues (2011) reported that the robotic procedure was associated with longer surgical time, longer postoperative use of nonsteroidal pain medications, and was more expensive than the laparoscopic approach. At 1 year, no differences in anatomic and functional outcomes have been reported, although the study was significantly underpowered to detect any differences in these outcomes; further evaluation of both laparoscopic and robotic approaches is required. Table 7-1 compares the advantages and disadvantages of the open, laparoscopic, and robotic sacral colpopexy.
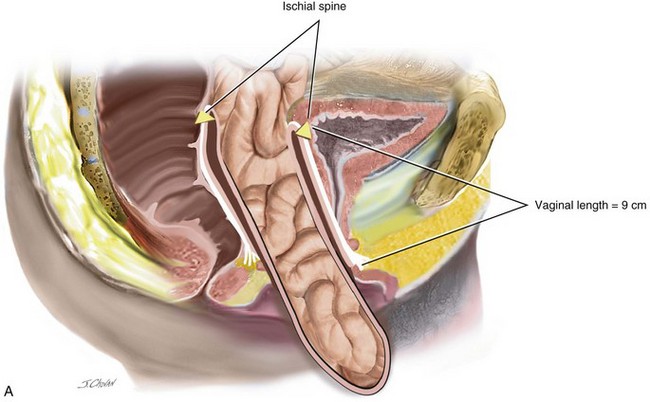
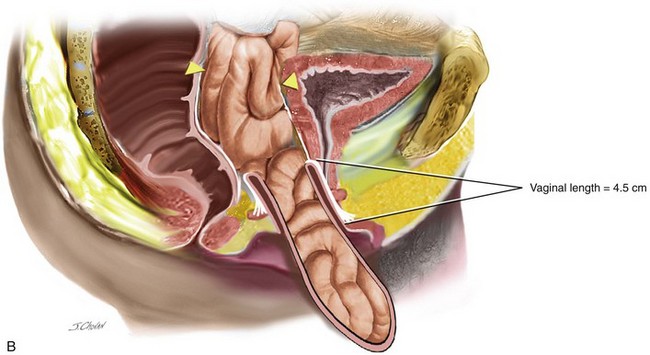
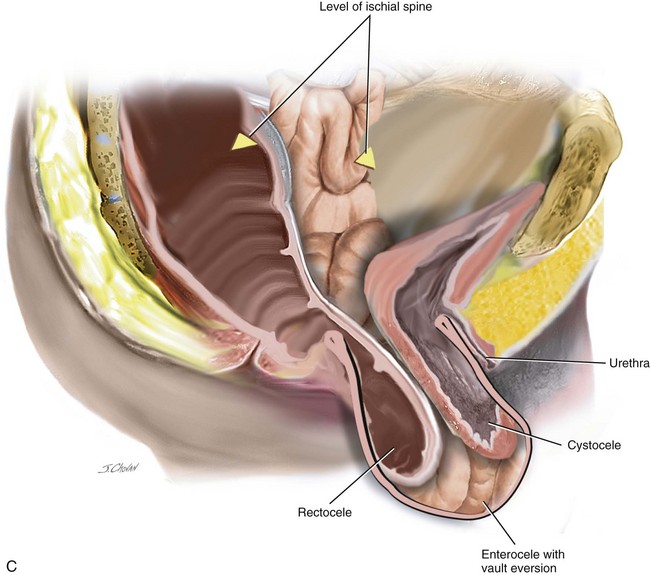
When isolated, mild apical ( descent to midpoint vagina) requires surgery vaginal hysterectomy with culdoplasty (see Chapter 4) or vaginal enterocele repair ( see chapter 9) is usually sufficient to provide a vagina of adequate length. As more of the anterior and or posterior vaginal walls are everted, the more complex the repair becomes. Usually, a more formal apical repair in conjunction with a repair of the anterior and/or posterior vaginal wall is required (Figure 7-2). The goal of any apical suspension should be to provide a high durable suspension of the apex without creating a significant distortion to the vaginal axis. In general, if the apex can be suspended to the level of the ischial spine, then the vaginal length will be approximately 9 cm.
Case 1: Laparoscopic Sacral Colpopexy
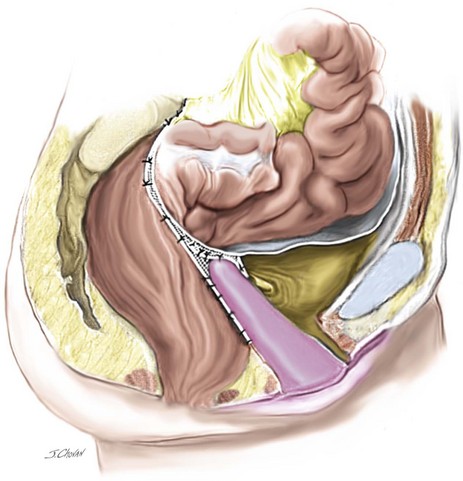
A 56-year-old patient has posthysterectomy vaginal vault prolapse and urinary incontinence. Her previous surgeries included vaginal hysterectomy and repair and an appendectomy. An examination confirms anterior compartment at the introitus and the vault extending 3 cm beyond the introitus. Urodynamic testing demonstrates a stable bladder and significant urinary stress incontinence with no voiding dysfunction. This patient consents to an LSC and colposuspension (Figures 7-3, 7-4, and 7-5). Figure 7-6 shows a sagittal view.
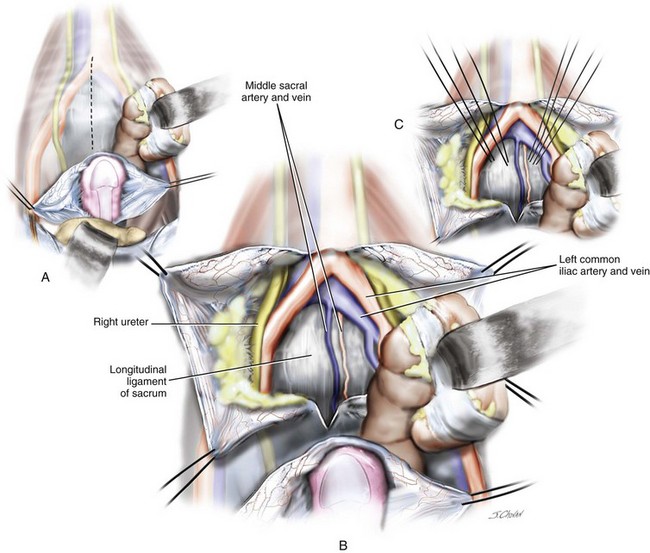
Figure 7-4 Anatomy of the sacral promontory.
(From Baggish MS, Karram MM: Atlas of pelvic anatomy and gynecologic surgery, ed 3, St Louis, 2011, Elsevier.)
Surgical Steps: Laparoscopic Sacral Colpopexy
1. Trocar placement: Figure 7-7 illustrates the anterior abdominal wall vasculature and preferred trocar placement. A 10-mm re-useable Hassan trocar is placed subumbilically under direct vision. Secondary trocars are introduced under laparoscopic vision with three further trocars used:
2. Figure 7-7 highlights the common trocar positions in relation to the anterior abdominal wall vessels. In patients with a prior vertical midline incision, a Palmer’s point entry technique can be used to minimize the risk of bowel perforation entry. (See Video 7-1 ![]() for a demonstration of the laparoscopic entry technique.)
for a demonstration of the laparoscopic entry technique.)
3. Steep Trendelenburg position facilitates the emptying of the small and large bowels from the pelvis.
4. Any adhesions present should be mobilized; significant adhesiolysis, defined as greater than 45 minutes, occurs in 25% of posthysterectomy cases. (See Video 7-2, “Laparoscopic Adhesiolysis before Laparoscopic Colpopexy,” ![]() for a video demonstration of laparoscopic adhesiolysis in three patients undergoing laparoscopy sacral colpopexy.)
for a video demonstration of laparoscopic adhesiolysis in three patients undergoing laparoscopy sacral colpopexy.)
5. The peritoneum is opened from the sacral promontory to the vault with unipolar scissors, staying medial to the uterosacral ligament and lateral to the large bowel. The scissors are in the left upper trocar, and nontraumatic forceps retract the peritoneum from the left and right lower trocars.
6. Once the dissection is at the vault, a nonheat and energy-conducting Apple vaginal probe is introduced into the vagina. The probe features blunt polymer tips for atraumatic use, surgical-grade stainless steel shafts, and color-coded anodized aluminum handles; it offers counter traction that facilitates the dissection of the bladder and bowel from the vagina.
7. For anterior dissection, atraumatic forceps in the left lower trocar reflects the bladder, and sharp dissection of the bladder from the vagina is performed with scissors in the left upper trocar. The dissection continues centrally to the level of the trigone, which is identified when the bladder will no longer mobilize from the vagina at approximately 7 cm along the anterior wall dissection.
8. Rectovaginal space is created with the vaginal probe acutely anteverted and atraumatic forceps in the left lower trocar, reflecting the bowel inferiorly, and let upper trocar space to approximately 8 cm along the posterior vaginal wall with the minimal use of electrosurgical energy and dissection.
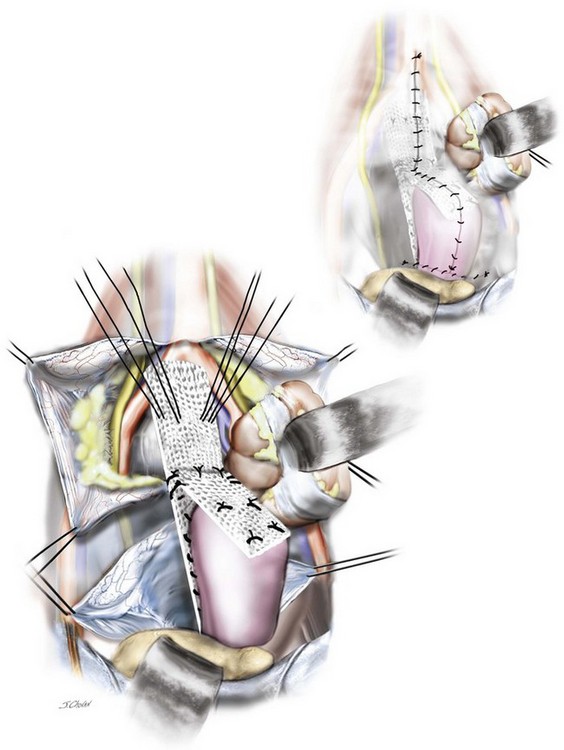
9. The mesh is prepared as demonstrated in Video 7-3 A 15 × 15 cm sheet of Ultrapro (JJ Ethicon) mesh is cut on the diagonal to create a posterior leaf of mesh approximately 5 to 6 cm wide and 22 cm long. A second anterior leaf is harvested approximately 5 to 6 cm wide and 8 cm long. These are sutured together using interrupted 2.0 polydioxanone suture (PDS).
10. The anterior mesh is secured to the vagina with two to three sutures (2.0 PDS) commencing with distal sutures and working toward the vault. The vaginal is then acutely anteverted, and the posterior leaf is secured to the vagina with two to three sutures (2.0 PDS) commencing distally and working toward the vault.
11. The vaginal probe is removed, and the posterior tail of the mesh is secured to the sacral promontory with nonabsorbable hernia tacks. Excellent exposure of the ligament is required to avoid injury to the surrounding vasculature. The potential advantages of the tacks over sutures are that they are hemostatic and can be easily inserted.
12. The retroperitoneum is closed with a continuous 2.0 PDS to ensure that no mesh is visible intraperitoneally at the completion of the surgery. Using this technique, virtually no bowel obstruction or symptoms of stasis occur, which seem common in some sacral colpopexy series.
13. Steep Trendelenburg position can be reduced, and the retropubic space is then entered if continence or prolapse surgery is to be performed.
14. Cystoscopic examination is performed to ensure that the ureters are patent and the bladder mucosa is intact.
15. Posterior colpoperineorrhaphy is usually required to complete the procedure.
16. The indwelling catheter is removed the next morning (6:00 AM), and a trial of void completed.
17. Antibiotics are initiated, thromboembolic stockings are worn for 7 days, and self-administered enoxaparin sodium (Clexane) (20 mg) is injected daily for 5 days for all patients. (See Video 7-3, “Laparoscopic Sacral Colpopexy” ![]() and Video 7-4, “Laparoscopic Colposuspension”
and Video 7-4, “Laparoscopic Colposuspension” ![]() for demonstrations of LSC and laparoscopic colposuspension.)
for demonstrations of LSC and laparoscopic colposuspension.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree