Fig. 37.1
Large filling defect on cross-sectional contrast enhanced CT urography that was proven transitional cell carcinoma
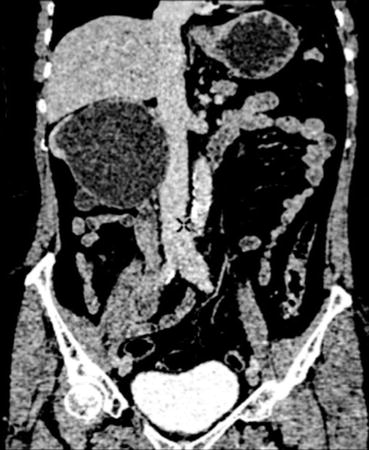
Fig. 37.2
Grossly hydronephrotic right kidney with a thin rim of cortex on contrast-enhanced CT Scan. Note a large filling defect in the upper ureter on the same side
Endoscopic Evaluation
Direct visualisation with ureteroscopy and biopsy were previously reserved for patients with equivocal finding on CT. However with increasing availability of fibroptic flexible urtereroscopes and endoscopic management option in selected group of patients, few centres routinely perform ureteroscopic biopsies irrespective of CT findings. The diagnostic accuracy of ureteroscopic visualisation and biopsies remains variable [23–27] (Table 37.1).
Table 37.1
Studies showing the diagnostic accuracy of ureteroscopic biopsies in the management of upper tract transitional cell carcinoma
Author | True positive | True –ve | False + ve | False −ve |
|---|---|---|---|---|
Guarnizo et al. (2000) [28] | 40/45 (89 %) | 0 | 5 | 0 |
Williams et al. (2008) [23] | 29/30 (97 %) | 0 | 2 | 0 |
Keeley et al. (1997) [29] | 40/42 (95 %) | 0 | 2 | 0 |
Chitale et al. (2008) [27] | 14/19 (74 %) | 0 | 0 | 0 |
Matsumoto et al. (2006) [26] | 33/35 (94 %) | 35 | 2 | 6 |
Shiraishi et al. (2006) [25] | 27/29 (93 %) | 1 | 0 | 5 |
Skolarikos et al. (2003) [24] | 62 | 2 | 0 | 0 |
A number of challenges remain unresolved with ureteroscopic biopsies such as lack of standardisation of biopsy technique, adequacy of specimen, type of preservative and impact on clinical management. Table 37.2 shows numbers of inadequate biopsies reported in the literature [24–29].
Table 37.2
Problem of inadequate ureteroscopic biopsies in the management of upper tract Urothelial carcinoma
Study | Study period | Biopsy technique/instruments | Number of inadequate biopsies as observe by reporting pathologists |
|---|---|---|---|
Chitale et al. [27] | 1994–2004 | Not documented | None mentioned |
Keeley et al. [29] | 1985–1995 | Basket/cup forceps | Not mentioned |
Guarnizo et al. [28] | 1990–1998 | 3 F cold cup biopsy forceps/11.5 F resectoscope | 5 (11 %) |
Matsumoto et al. [26] | 1998–2004 | 3 F cold cup biopsy forceps | 4 (6 %) |
Shiraishi et al. [25] | 1995–2001 | 3 F cold cup biopsy forceps | 5 (12.5 %) |
Skolarikos et al. [24] | 1989–2001 | 3 F cold cup biopsy forceps | 11 (15 %) |
Williams et al. [23] | 1998–2006 | 3 F cold cup biopsy forceps | 0 |
The accuracy of ureteroscopic biopsies can be enhanced by the application of blue light with oral 5-Aminolevulinic acid or narrow band imaging particularly for flat lesions such as carcinoma in situ [30]. Ureteroscopy enables histological confirmation with biopsies and could be taken as a further evaluation with retrograde studies. Ureteroscopic grade evaluation of biopsy correlates well with resected specimen; however the stage correlation is poor [31].
Urinary Biomarkers
There is currently increasing interest in the development of a reliable urinary biomarker in the diagnosis of UUT-UCC. They have potential role in the diagnosis as well as follow up management. Urinary cytology has poor sensitivity particularly for low grade lesion and is influenced by the experience of the examining pathologist. Its sensitivity is improved by direct ureteroscopic collection by washings or brush cytology. Despite this only a poor sensitivity of 60–70 % could be achieved at best [30]. A number of new urinary biomarkers are currently being investigated. UroVysion fluorescence in situ hybridization (FISH) has shown the most promising results with sensitivity and specificity reports of 52–100 % and 80–100 % respectively in the detection of upper tract tumours (Table 37.3) [30, 32–35].
Staging
Unlike bladder cancer 60 % of upper tract tumours are invasive at the time of diagnosis. Spread is direct, lymphatic or by haematogenous route. The lymphatic spread is to paraortic, paracaval, common iliac and pelvic lymph nodes. Haematogenous metastasis can occur to liver, lungs and bone. Staging is based on the TNM classification [36] (Table 37.4).
Table 37.4
TNM classification
Primary tumour (T) |
TX-Primary tumour cannot be assessed |
T0-No evidence of primary tumour |
Ta- Non-invasive papillary carcinoma |
Tis-Carcinoma in situ |
T1-Tumour invades subepithelial connective tissue |
T2-Tumour invades muscle |
T3-(For renal pelvis only)Tumour invades beyond muscularis into peripelvic fat or the renal parenchyma |
(For ureter only) Tumour invades beyond muscularis into periureteric fat |
T4-Tumour invades adjacent organs, or through the kidney into perinephric fat |
Regional lymph nodes (N) |
NX-Regional lymph nodes cannot be assessed |
N0-No regional lymph node metastasis |
N1-Metastasis in a single lymph node 2 cm or less in the greatest dimension |
N2-Metastasis in a single lymph node, ≤2 cm but not >5 cm in the greatest dimension or multiple lymph nodes, none >5 cm in greatest dimension |
N3-Metastasis in a lymph node more than 5 cm in greatest dimension |
Distant metastasis (M) |
M0-No distant metastasis |
M1-Distant metastasis |
Grading
Tumours are also graded often by both the 2004 and the more familiar 1973 WHO classifications [37].
1973 WHO Classification
G1 well differentiated
G2 moderately differentiated
G3 poorly differentiated
2004 WHO Classification
Papillary neoplasm of low malignant potential (PNLMP)
Low grade papillary carcinoma
High grade papillary carcinoma
Stage and grade at presentation dictate prognosis, with staging being the single most important prognostic indicator [38]. Other factors which are independent negative prognostic factors are advanced age, lymph vascular invasion, tumour necrosis, sessile tumour architecture and presence of concomitant CIS. Presence of tumor microsatellite instability (MSI) is a favourable prognostic factor [39].
Management of Localized UUT-UCa
Surgical excision or endoscopic ablations are the most common treatment modalities offered to patients with localised disease. Factors influencing treatment of localised UUT-UCa are renal function, function of contra-lateral kidney, availability of local expertise and associated co-morbidities. Broadly treatment options can be categorised into:
(i)
Radical nephroureterectomy
(ii)
Nephron/Ureter-sparing procedures
Radical Nephroureterectomy
Radical nephroureterectomy is the standard of care for many patients with localised disease. This procedure involves complete excision of the kidney (along with the perinephric fat and gerota’s fascia) and ureter. The nephroureterectomy is combined with an ipsilateral bladder cuff excision as the excised ureteric stump is a frequent site for recurrence. The procedure can be performed by open, laparoscopic and robotic approaches. The eventual approach employed is subject to local expertise availability, although laparoscopic approach is the favoured option in a majority of institutions.
Open Nephrourterectomy (ONU)
This is a significantly morbid procedure usually requiring two large incisions (can be performed with one long incision). Patients have considerable post operative analgesia requirement, prolonged post-operative stay and increased blood transfusion rates in comparison with minimally invasive approaches [40].
Laparoscopic Nephrourterectomy (LNU)
Laparoscopic Nephrourterectomy is increasingly becoming the gold standard procedure in a majority of institutions for localised UUT-UCC. It can be performed by transperitoneal, retroperitoneal and hand assisted approaches. LNU offers significantly better immediate functional outcomes and equivalent oncological outcomes when compared to an ONU.
Robotic Nephrourterectomy
Robotic approaches for various urological techniques have been in ascendancy in recent years. Current evidence on robotic assisted nephrourterectomy although sparse has shown promising immediate outcomes and short term oncological outcomes [41]. Cost, standardisation of technique and paucity in literature for long term oncological outcomes have limited their usage.
Open Versus Laparoscopic Nephroureterectomy
The only randomised control trial identified in a recent Cochrane review [41, 42] comparing the two approaches reported lesser blood loss (104 ml vs. 430 ml, P < 0.001) and mean time to discharge (2.30 days vs. 3.65 days, p < 0.001) in favour of LNU. At a median follow up of 44 months, the 5 year cancer-specific survival and 5 year metastasis-free survival rates were similar for the two groups. A non-systematic review reported that the laparoscopic approach was associated with a longer operating time (277 min vs. 200 min), but a reduced blood loss (241 ml vs. 463 ml), a reduced analgesic requirement, and a shorter hospital stay compared to open surgery.
A summary of Cochrane review identified multiple case series (retrospective and prospective) reporting outcomes of laparoscopic nephroureterectomy with variations regarding the use of laparoscopic approach (transperitoneal versus retroperitoneal), and management of the distal ureter. Compared with open surgery, literature suggests that laparoscopic surgery has various benefits with respect to postoperative analgesia requirements, hospitalization duration, cosmesis, and convalescence. With intermediate follow-up, cancer-related outcomes seem similar between the open and laparoscopic surgical modalities; however design, methodology and reporting of studies were poor.
Management of the Distal End of the Ureter- Techniques and Results
Adequate excision of the ipsilateral distal ureter and adjacent bladder cuff is an essential step of nephroureterectomy procedure. Failure to achieve this can result in recurrence rates of 34 % at the ipsilateral ureteric stump [43]. Various approaches to deal with the distal end of the ureter have been described (open intra and extra-vesical techniques, pluck, endoscopic ureteral detachment, ureteral intussusceptions, cystoscopic unroofing and laparoscopic stapling and extravesical laparoscopic excision). There is currently no agreement on most appropriate approach and current practices are based on personal preference and expertise available [41].
Long-Term Oncological Outcomes: LNU Verus ONU
Nephroureterectomy with ipsilateral bladder cuff excision regardless of what approach is used offer the best oncological outcomes. A non-systematic review comparing laparoscopic and open approaches showed no statistically significant differences between the two groups for bladder recurrence (24 % vs. 24.7 %), local recurrence (4.4 % vs. 6.3 %) and distant metastasis (15.5 % vs. 15.2 %). The 2 and 5-year survival rates for the laparoscopic and open groups were 75.2 % vs. 76.2 %, and 81.2 % vs. 61 %, respectively [40].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







