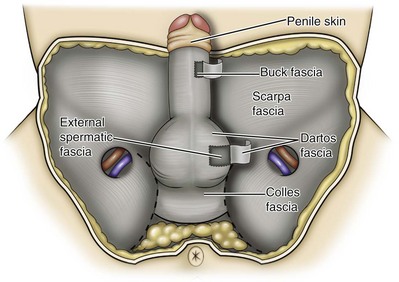
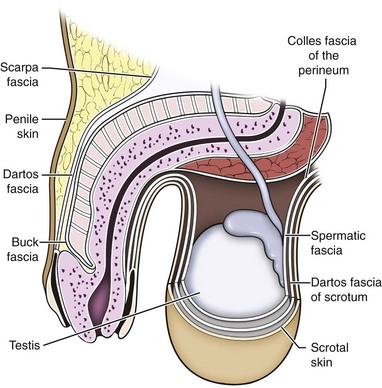
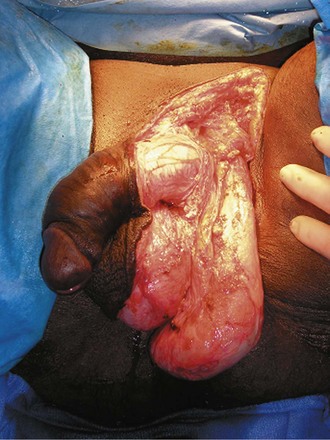
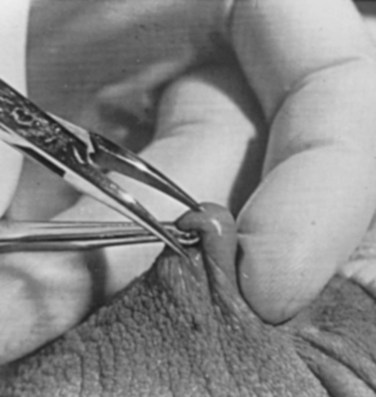
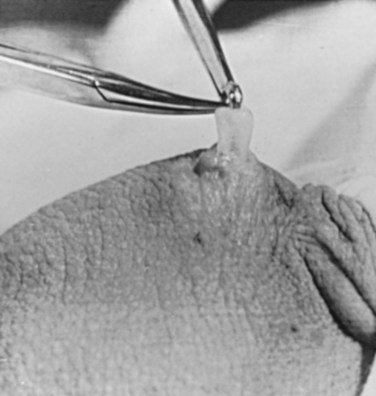
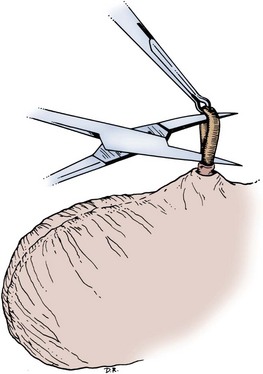
Parviz K. Kavoussi, MD, Raymond A. Costabile, MD The scrotum is a unique body component because the superficial anatomic location of the scrotum and scrotal contents facilitates physical examination, imaging, and surgical access. It is crucial to understand the blood supply to the organs within the scrotum when surgical intervention is indicated. This is summarized in Table 37–1. Table 37–1 Blood Supply to Testis, Epididymis, and Vas Deferens There is a predictable pathway for the spread of scrotal infections and postoperative fluids based on scrotal anatomy. This defined anatomy for the spread of infection is demonstrated in patients with Fournier gangrene (necrotizing fasciitis of the scrotum). Anatomic barriers to the spread of necrotizing fasciitis include the dartos fascia of the penis and scrotum, Colles fascia of the perineum, and Scarpa fascia of the anterior abdominal wall. The testes and epididymes tend to be spared from necrotizing fasciitis of the scrotum (Figs. 37-1 and 37-2) (Gupta et al, 2007). Figure 37–1 Anatomic barriers to the spread of infection. (Modified from Kavoussi PK, Costabile RA. Disorders of scrotal contents: orhcitis, epididymitis, testicular torsion, torsion of the appendages, and Fournier’s gangrene. In: Chapple CR, Steers WD, editors. Practical urology: essential principles and practice. London: Springer-Verlag; 2011.) Figure 37–2 Sagittal view of anatomic barriers to the spread of infection. (Modified from Kavoussi PK, Costabile RA. Disorders of scrotal contents: orhcitis, epididymitis, testicular torsion, torsion of the appendages, and Fournier’s gangrene. In: Chapple CR, Steers WD, editors. Practical urology: essential principles and practice. London: Springer-Verlag; 2011.) Effective anesthetic techniques for scrotal surgery range from local injection with or without sedation to spinal to general anesthesia. The use of a spermatic cord block with local infiltration of 0.5% xylocaine without epinephrine is a simple, cost-effective anesthetic technique that can be implemented by the surgeon for outpatient scrotal surgical procedures. Regional cord block can typically be performed without premedication with satisfactory patient analgesia (Magoha, 1998; Wakefield and Elewa, 1994). Spermatic cord block can be used in patients with large hydroceles for anesthesia by initially percutaneously draining the hydrocele, performing the block, followed by hydrocelectomy (Reale et al, 1998). Outpatient scrotal surgery performed with midazolam sedation and a local block, with sedation reversal at the end of the procedure has a very high patient satisfaction rate (Birch and Miller, 1994). The overall infection rate with scrotal surgery is relatively low, ranging from zero to ten percent. There is no difference in the incidence of postoperative wound infections or complications in patients undergoing hydrocelectomy or spermatocelectomy when comparing iodine-based versus chlorhexidine antiseptic preparations. Scrotal cases are considered as class II (clean-contaminated surgeries), which makes it reasonable to use preoperative antibiotics (Kiddoo et al, 2004). The American Urological Association (AUA) best practice policy statement on urologic surgery antimicrobial prophylaxis recommends a single dose of preoperative antibiotics if the patient has risk factors for infection, including advanced age, anatomic anomalies of the urinary tract, poor nutritional status, smoking, chronic corticosteroid use, immunodeficiency, externalized catheters, colonized endogenous/exogenous material, a distant coexistent infection, or prolonged hospitalization. The recommended antibiotic prophylaxis is a dose of a first generation cephalosporin, or clindamycin as an alternative antimicrobial (Wolf et al, 2008). Patients who underwent preoperative clipping for hair removal the morning of surgery had a significantly lower wound infection rate than those who underwent shaving or clipping the night before surgery (Alexander et al, 1983). Patients with multiple scrotal cysts can be managed with surgical excision with excellent cosmetic results and low recurrence rates (Noël et al, 2006). The classical management of scrotal sebaceous cysts is surgical excision with excellent outcomes and minimal morbidity with good cosmetic results. Less invasive techniques, such as Nd : YAG photocoagulation, have been performed successfully but are not considered standard management (Franco de Castro et al, 2002). Partial scrotectomy is an uncommon procedure. Partial scrotectomy is most commonly performed with infectious processes such as Fournier gangrene. Partial scrotectomy has been advocated after trans-scrotal exploration, orchiectomy, biopsy, or when aspiration has been performed for a scrotal mass and the pathology has revealed a nonseminomatous germ cell tumor of the testis. Prompt and aggressive management has resulted in no local recurrences due to scrotal tumor contamination, even when a tumor was found in the scrotectomy specimen (Johnson and Babaian, 1980; Boileau and Steers, 1984; Leibovitch et al, 1995). In a small group that underwent aggressive local surgical resection and did not receive adjuvant chemotherapy, there was no increase in the local or distant recurrence (Giguere, 1988). Total scrotectomy is less commonly performed than partial scrotectomy. Total scrotectomy is often necessary when there is extensive involvement of the scrotum with Fournier gangrene. Total scrotectomy has also been described for radical oncologic procedures, concomitantly with cystoprostatectomy, penectomy, or pelvic exenteration with aggressive cases of squamous cell carcinoma of the prostate (Sarma et al, 1991). Treatment of Fournier gangrene should include emergent radical surgical debridement and intravenous broad-spectrum antibiotics. When culture results are available the antibiotics can be tailored to the organisms based on sensitivities. Treatment should be performed expeditiously and aggressively, because Fournier gangrene is a life-threatening process. All nonviable and necrotic tissue must be aggressively excised (Fig. 37–3). An empirical broad-spectrum antibiotic regimen for the initial treatment of Fournier gangrene includes a third-generation cephalosporin, an aminoglycoside (if the creatinine clearance is acceptable), and metronidazole. Aggressive fluid resuscitation is required, including the use of blood and blood products when needed. After debridement, adequate nutrition with early enteral feeding, when possible, is crucial for wound healing. Repeat debridement should be performed two days after the initial exploration to excise any remaining nonviable tissue. Multiple resections may be necessary. If the source of the infection is anorectal or the wound is contaminated, a colostomy may need to be performed to divert fecal flow (Ghnnam, 2008). Similarly, patients may require cystostomies for urinary diversion when there is a urinary source exacerbating the necrotizing fasciitis. Figure 37–3 Aggressive debridement of Fournier gangrene. (From Kavoussi PK, Costabile RA. Disorders of scrotal contents: orhcitis, epididymitis, testicular torsion, torsion of the appendages, and Fournier’s gangrene. In: Chapple CR, Steers WD, editors. Practical urology: essential principles and practice. London: Springer-Verlag; 2011.) Once the patient has been initially treated and resuscitated and all necrotic tissue has been excised, most wounds can be closed secondarily. Large wounds will often require skin grafts for coverage. Fasciocutaneous rotational thigh flaps may be used for coverage with good cosmetic results (Bhatnagar et al, 2008). Wound closure is performed as soon as there is no evidence of infection or remaining necrotic tissue, and there is a viable bed that will allow reapproximation or grafting (Ghnnam, 2008). Patients with less than 50% scrotal skin loss can almost always be closed primarily without major difficulty. The testes may be placed in thigh pouches until the time of definitive reconstruction in cases with major scrotal skin loss (Gudaviciene and Milonas, 2008). Vacuum-assisted closure devices (Wound V.A.C.) have been used to help these complex wounds heal after wide excision and debridement. This technique has been shown to be as effective as conventional wound care in healing wounds. These patients require fewer dressing changes, have less pain, fewer skipped meals, and greater mobility (Ozturk et al, 2009). The use of a small intestinal submucosa graft and fibrin sealant is an option for closure of scrotal defects after excision for Fournier gangrene when standard grafting is not possible (Kavoussi and Bird, 2007). A severity index has been created and validated to identify prognostic factors in patients suffering from Fournier gangrene. Parameters associated with mortality include abnormalities in heart rate, respiratory rate, serum creatinine, serum bicarbonate, serum lactate, and serum calcium. There is a 46% mortality rate in patients with a severity index score of nine or greater, and a 96% survival rate in patients with a severity index score of less than nine. Necrotizing fasciitis involving the abdominal wall or the lower extremities is associated with increased mortality (Corcoran et al, 2008). Vasectomy is a highly effective and safe form of contraception (Schwingl and Guess, 2000). Vasectomy was first described by Sir Ashley Cooper in the United Kingdom when he did experiments to vasectomize dogs (Cooper, 1827). Approximately 526,501 men undergo vasectomy annually in the United States, which makes vasectomy the most commonly performed urologic surgical procedure. 0.01% of men between the ages of 25 and 49 undergo vasectomy annually (Barone et al, 2006). This comprises 11% of all married couples. EMLA (emulsion of lidocaine and prilocaine) cream applied as topical anesthesia on the scrotal skin one hour prior to injection of 1% lidocaine followed by vasectomy does not decrease the pain associated with vasectomy compared with the use of injectable anesthesia alone (Thomas et al, 2008). The no-needle jet anesthetic technique has been described to eliminate the needle for anesthetic injection. This technique uses the MadaJet medical injector (MADA Medical Products, Carlstadt, NJ) to deliver lidocaine without epinephrine by means of a high-pressure injector through a tiny head to beneath the skin to diffuse a mist of anesthetic around the vas (Weiss and Li, 2005). Vasectomy should be performed in a warm room with warm preparation solution to allow scrotal relaxation, regardless of the technique employed. Shaving should be performed prior to the procedure to minimize the risk of infection. There is no difference in the rate of postvasectomy infection in men randomized to prophylactic antibiotics versus no antibiotics; therefore antibiotic prophylaxis is not recommended (Khan, 1978). In any chosen technique, the use of a single incision or bilateral scrotal incisions is based on surgeon preference. Many surgeons advocate bilateral scrotal incisions to minimize the risk of dividing the same side twice and to allow performing the vasectomy at a position in the midportion of the vas deferens. After induction of adequate local anesthesia, an incision is made over the isolated vas deferens, which is grasped tightly between the thumb and middle fingers. The vasal sheath is sharply divided down to the vas. The vas is delivered through the incision; the deferential artery, nerves, veins, and adjacent tissue are separated from the vas, and the vas is divided. Some surgeons remove a small segment of vas deferens, although most urologists who perform vasectomy reversals prefer not to, which allows easier future reversal. The AUA Policy Statement (American Urological Association, 2007) states that removal of a segment of vas deferens for histologic confirmation is not required or recommended. Most surgeons occlude both the testicular and abdominal ends of the vas with suture ligation, hemoclips, intraluminal fulguration with electrocautery, or fascial interposition. These techniques are further discussed below. The same procedure is repeated on the contralateral vas deferens. The no-scalpel vasectomy was initially described in China in 1974 (Li, 1976). Routine antibiotic use is not needed for vasectomy patients undergoing no-scalpel vasectomy with sterile technique (Seenu and Hafiz, 2005). The no-scalpel technique significantly decreases the rate of hematomas, infections, and pain during the procedure. Patients who undergo the no-scalpel technique also resume sexual activity sooner after surgery and have a shorter operative time than occurs with the conventional technique (Sokal et al, 1999, Cook et al, 2007a). There are several variations to the technique of the no-scalpel vasectomy. One technique involves firmly securing the vas through the skin with a ring-tipped vas deferens fixation clamp (Fig. 37–4) after local anesthesia has been administered as described above (Fig. 37–5). A modified, sharpened tipped curved hemostat (Fig. 37–6) is used to puncture the skin and the vas sheath, and the hemostat is spread to stretch the hole that is made. Then the vas deferens is lifted out, divided, the occlusion technique of choice is employed, inspection is done for hemostasis, and the vas deferens is replaced in the scrotum. The same procedure is performed on the contralateral vas deferens (Huber, 1988). The perforation in the skin can be closed with an absorbable suture, but can also be left open, and will heal well without closure. Figure 37–4 Ring-tipped vas deferens fixation clamp. Cantilevered design prevents injury. (From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.) (From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.) Figure 37–6 Sharp, curved mosquito hemostat. (From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.) A variation of this technique employs local anesthesia; fixes the vas through the scrotal skin with the ring-tipped vas deferens fixation clamp; and pierces the scrotal skin, vas sheath, and vas deferens in the midline with a sharpened tipped curved hemostat held at 45 degrees from horizontal (Fig. 37–7). To prepare the vas deferens for division, the clamp is then rotated 180 degrees relative to the pierced vas deferens. The remainder of the procedure is performed in the same manner as described above, and this is done bilaterally (Schlegel and Goldstein, 1992). Figure 37–7 Puncture of the skin, vas sheath, and wall into the lumen. (From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.) Another technique for performing no-scalpel vasectomy is to isolate the vas deferens after induction of adequate local anesthesia, grasping it tightly between the thumb and middle finger, and puncture the skin overlying the vas deferens with a sharpened tipped curved hemostat. The curved hemostat is used to spread the skin to enlarge the vertical slit in the skin just large enough to allow the ring-tipped vas deferens fixation clamp to fit through to grasp the vas deferens. The vas deferens is grasped and brought up through the puncture, and the vasal fascia and vessels can be spread off the vas deferens to expose the bare vas deferens by spreading the sharp-tipped curved hemostat onto the vasal fascia to open the vasal fascia (Figs. 37-8 and 37-9). The remainder of the vasectomy is performed as described above for bilateral procedure (Li et al, 1991). This modification of making the puncture prior to grasping the vas deferens with the ring-tipped vas deferens fixation clamp was found to significantly decrease the operative time and showed no difference in incision length, postoperative pain, or time to return to work in a randomized prospective evaluation (Chen et al, 2005). Figure 37–8 Delivery of the clean vas. (From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.) Figure 37–9 Segment cleaned. (From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.) There have been concerns about the risk of vasal necrosis and sloughing distal to the ligated end when suture ligation is performed, theoretically increasing the risk of recanalization. Low-voltage thermal occlusion with intraluminal electrocautery in the abdominal and testicular ends of the divided vas deferens reduces recanalization rates to less than 0.5% (Schmidt, 1987; Barone et al, 2004). Vasectomy failure rates have been reported to be less than 1% when both the testicular and abdominal ends of the divided vas deferens are occluded with hemoclips (Moss, 1974; Bennett, 1976). Interposition of dartos fascia between the divided ends of the vas deferens is another technique for occlusion. This method has been reported to reduce the recanalization rate even further, nearly to zero (Esho and Cass, 1978; Sokal et al, 2004). Percutaneous vasectomy has been performed on over 500,000 men in China. This technique employs chemical occlusion by fixing the vas deferens up to the scrotal skin tightly, puncturing the lumen of the vas deferens with a 22-gauge needle, and then cannulating the lumen of the vas deferens with a 24-gauge blunt needle. For confirmation of vas deferens cannulation, Congo red is injected into the lumen of the abdominal end of the right vas deferens, and methylene blue is injected into the lumen of the left vas deferens prior to chemical occlusion by injection of 20 µl of two parts phenol to one part N-butyl-2-cyanoacrylate mixture. Following chemical occlusion the patient should void. If the urine is red, the left side was not cannulated, if it is blue, the right side was not cannulated, and if it is brown, that indicates bilateral successful cannulation (Ban, 1980; Li, 1980). Although these chemicals are not approved for use in the United States by the Food and Drug Administration, they appear to be safe by toxicologic testing and experience in China. Fascial interposition is the technique that has been found to decrease vasectomy recanalization rates the most significantly. Randomized controlled trials examining the other techniques are not available. Several trials have been performed using irrigation of the abdominal ends of the vas deferens with saline, but there was no difference in time to azoospermia (Cook et al, 2007b). Open-ended vasectomy, where the testicular portion of the vas deferens remains patent, is another technique that has been evaluated with the aim of decreasing epididymal pressure by performing intraluminal cautery or another method of occlusion on the abdominal end, while leaving the testicular end unoccluded. Ninety-seven percent of patients undergoing open-ended vasectomy develop sperm granulomas. This reduces pressure-induced damage to the epididymis, but increases the vasectomy failure rate to as high as 7% to 50% (Shapiro and Silber, 1979; Goldstein, 1983). There is a significant decrease in the failure rate with open-ended vasectomy when fascial interposition is performed, decreasing the failure rate by approximately 7% (Li et al, 1994). Technical aspects of performing vasectomy can affect the ease of microsurgical vasectomy reversal in the future if needed (Mammen et al, 2008). One procedural aspect is that excising a lengthy (>1 cm) segment of vas deferens is associated with the need for a higher scrotal incision, possibly up to the lower inguinal region, with the potential for anastomotic tension with microscopic vasectomy reversal. Vasectomy reversal can be far more difficult when a lengthy portion of the vas deferens has been excised, with concomitant increases in operative time, length of incision, and postoperative pain (Practice Committee of the American Society for Reproductive Medicine, 2006). Another procedural aspect is the location along the length of the vas deferens where the vasectomy is performed. Experts in microsurgery agree that the anastomosis is least problematic when the lumen of the vas deferens is largest and most concentric, as opposed to the lumen in the epididymis or the convoluted vas (Mammen et al, 2008). Prospective studies show that the length of the testicular vas deferens present at the time of reversal has a direct correlation with the presence of seminal fluid containing intact sperm at the time of microscopic vasectomy reversal. A testicular length of vas deferens less than 2.7 cm correlates with seminal fluid without intact sperm 85% of the time, and testicular length over 2.7 cm is associated with intact sperm in seminal fluid 94% of the time. For each centimeter increase in testicular remnant length, the probability of whole sperm being present increases fourfold (Witt et al, 1994). Division of the vas deferens should be performed approximately 3 cm distal to the cauda of the epididymis in the straight portion of the vas deferens at the time of vasectomy. The other technical aspect to consider is the occlusion technique employed. All occlusive modalities for vasectomy carry a similarly high efficacy in terms of postprocedure azoospermia. To date, no specific studies on occlusion technique as a predictor of reversal success have been performed. Simple transection of the vas deferens followed by low-voltage intraluminal cautery occlusion followed by fascial interposition provides successful vasectomy and may result in minimal inflammatory reaction. It would stand to reason that minimizing inflammation near the vas deferens would provide the optimum condition for microscopic vasectomy reversal in the future (Mammen et al, 2008). There is no vasectomy technique that is 100% effective, and a definitive standard to declare a patient sterile has not been agreed upon. Time to reach azoospermia is variable, although over 80% of patients achieve azoospermia by three months and after twenty ejaculations. Persistent nonmotile sperm are present in 1.4% of postvasectomy patients. This data points to obtaining a semen analysis at three months and twenty ejaculations after vasectomy to reveal azoospermia. If the semen analysis does not show azoospermia, periodic semen analyses can be obtained until azoospermia is achieved. Patients who have small numbers of persistent nonmotile sperm can be advised to cautiously discontinue contraception (Griffin et al, 2005). There is evidence that these men will ultimately reach azoospermia. Vasectomy should be repeated if any motile sperm are found in the ejaculate three months after the initial vasectomy (Aradhya et al, 2005). An immunodiagnostic test, the SpermCheck Vasectomy (ContraVac, Charlottesville, VA), has been developed to allow patients to test themselves for severe oligospermia or azoospermia at home following vasectomy. This was developed to increase compliance with postvasectomy evaluation of semen parameters. The SpermCheck Vasectomy test was 96% accurate at predicting whether sperm counts were greater or less than a threshold of 250,000 sperm per milliliter (Klotz et al, 2008). Local complications of vasectomy include hematoma, infection, Fournier gangrene, chronic scrotal pain, and traumatic fistula/scrotal sinus (Awsare et al, 2005). Surgeon volume and experience is the most important predictor of postoperative complications (Kendrick et al, 1987). Hematoma is the most common complication of vasectomy. The rate of hematoma formation after vasectomy ranges between 0.09% and 29%, with a mean incidence of 2% (Kendrick et al, 1987). The no-scalpel technique has decreased the hematoma rate to a 0.5% incidence (Pant et al, 2007). The rate of infection from vasectomy with the conventional technique was reported between 12% and 38%, but decreased to 0.4% with the no-scalpel technique (Appell and Evans, 1980; Pant et al, 2007). Although it is an exceedingly rare complication, Fournier gangrene has been reported as a complication in men undergoing vasectomy (de Diego Rodríguez et al, 2000; Romero Pérez et al, 2004). Up to 30% of men have short-term scrotal pain lasting a few weeks. However, postvasectomy pain syndrome, or long-term scrotal pain after vasectomy, occurs in approximately one in 1000 vasectomies, although some report the incidence to be as high as 15% (McConaghy et al, 1996; Awsare et al, 2005; Tandon and Sabanegh, 2008). Postvasectomy pain syndrome has no association with immediate postoperative complications such as hematoma or infection. There are several theories about the cause of postvasectomy pain syndrome. One is that dilation of the epididymal duct with obstruction of the testicular end of the vas deferens produces interstitial fibrosis. Another theory is that extravasation of spermatozoa, with epididymal duct rupture forming a sperm granuloma at the site where the vas deferens is transected, results in perineural fibrosis and inflammation, because sperm are highly antigenic (McMahon et al, 1992). This contradicts the previous belief that sperm granulomas are protective against postvasectomy pain syndrome by relieving pressure, although most sperm granulomas are asymptomatic (Tandon and Sebanegh, 2008). Interestingly, there are markedly increased pressures in the epididymis and the testicular end of the vas deferens after vasectomy, but these pressures were not found to be transmitted to the seminiferous tubules in human micropuncture studies (Johnson and Howards, 1975). It is unclear why some patients would develop long-term symptoms and others transient ones. Factors such as age, socioeconomic status, race, environmental, or vasectomy technique have not identified patients at risk for postvasectomy pain syndrome (Tandon and Sebanegh, 2008). Conservative therapy should be the first line of therapy. This includes scrotal elevation and support, heat or ice (as needed for comfort), and nonsteroidal anti-inflammatory drugs. Empiric antibiotic therapy is not recommended without evidence of infection (Selikowitz and Schned, 1985). Conservative therapy should be employed for at least three months for postvasectomy pain. Spermatic cord blocks should be considered after failure of conservative therapy. Surgical therapy might be considered if the above methods fail. When pain is clearly localized to a sperm granuloma, excision of the granuloma and intraluminal cautery occlusion of the vas deferens may relieve the pain and prevent recurrence (Schmidt, 1979). Epididymectomy has been performed in patients who had point tenderness to the epididymis, and epididymal dilation after vasectomy and failed conservative therapy. Predictors of poor outcomes with epididymectomy are atypical symptoms, concomitant erectile dysfunction, and a normal-appearing epididymis on scrotal ultrasound (West et al, 2000). Up to 50% of patients who were properly selected for epididymectomy were cured of postvasectomy pain syndrome (Chen and Ball, 1991). The patient must consider that vasectomy reversal will no longer be feasible after epididymectomy. Vasectomy reversal rendered 69% of patients with postvasectomy pain syndrome pain free (Nangia et al, 2000). Although it has only been evaluated in a small number of patients, microscopic denervation of the spermatic cord in patients who had temporary relief with a spermatic cord block resulted in complete pain relief in 76% and improvement in pain in 9% of these men (Levine and Matkov, 2001). The last resort consideration after failure of conservative and more invasive interventions have failed, is orchiectomy for severe intractable pain after vasectomy. In this group, pain relief was reported in 73% of men who underwent inguinal orchiectomy versus 55% in those who underwent scrotal orchiectomy for postvasectomy pain syndrome (Davis et al, 1990). There is a report of 0.3% rate of scrotal sinus/vasocutaneous fistula after no-scalpel vasectomy (Pant et al, 2007). Previous studies found an increased risk of prostate cancer in men who underwent vasectomy (Giovannucci et al, 1993). Detection bias is thought to be the source of this association of prostate cancer with vasectomy (Millard, 1999). Recent investigations have found that there is no association between vasectomy and prostate cancer (Schuman et al, 1993; Holt et al, 2008). There is no association between vasectomy and prostate cancer in developing countries in which there are low incidences of prostate cancer in the general population as well (Schwingl et al, 2009). Screening recommendations for prostate cancer should be no different in men who have undergone vasectomy than those who have not undergone vasectomy (Healy, 1993). Vasectomy does not place the patient at an increased long-term risk for cardiovascular disease or atherosclerosis (Coady et al, 2002; Goldacre et al, 2005). Previous studies suggested that vasectomy may be a risk factor for patients with primary progressive aphasia, a dementia syndrome with aphasia as the presenting symptom (Weintraub et al, 2006). There are no longitudinal studies confirming this, and there have been many large, epidemiological studies comparing vasectomized and nonvasectomized men without any increased risk of dementia. There is no evidence that vasectomy adversely affects psychological health status (Thonneau and D’Isle, 1990). There is a disruption of the blood-testis barrier when vasectomy is performed. Of men who undergo vasectomy, 60% to 80% of them have detectable levels of antisperm antibodies in the serum (Fuchs and Alexander, 1983). After vasectomy, 50% to 60% of men develop sperm agglutinating antibodies, and 20% to 30% develop sperm immobilizing antibodies (Kovacs and Frances, 1983). Although some studies suggest that antisperm antibodies persist, others suggest that they diminish in two or more years after vasectomy, but neither immune complex deposition nor circulation are increased in vasectomized men (Witkin et al, 1982). A spermatocele is a cystic dilation of an epididymal tubule, which is benign in nature. Spermatoceles are very common, occurring incidentally in 30% of men on high-resolution ultrasound, and they are typically asymptomatic and do not cause epididymal obstruction, and therefore rarely require intervention. Men typically seek surgical treatment when the spermatocele has reached the approximate size of the testis and is causing pain with point tenderness (Walsh et al, 2007). Surgical treatment for chronic epididymitis is poorly studied in clinical trials with no level 1 evidence to support the use of a specific surgical procedure. In one study, ten patients with chronic epididymitis (defined as epididymal pain lasting greater than 3 months), underwent epididymectomy for intractable symptoms. Only one of these patients had significant improvement in pain (Davis et al, 1990). Other authors have reported much higher success rates, such as six out of seven patients (86%) having significant improvement in pain after epididymectomy (Chen and Ball, 1991). Chronic or recurrent epididymitis and persistent epididymalgia with point tenderness to the epididymis may be reasonable indications for epididymectomy (Padmore et al, 1996). Surgical treatment for chronic epididymitis should only be considered after failure of extensive conservative therapy and after appropriate counseling, with the understanding that the symptoms may not improve after surgery, or may indeed worsen. A retrospective review of men who underwent epididymectomy for chronic epididymitis showed that outcomes were best when the patient had a palpable epididymal abnormality on physical examination. Men in this study without a palpable abnormality, but with sonographic changes, had slightly worse outcomes, and those with neither a palpable abnormality nor a demonstrable ultrasound abnormality did not improve with epididymectomy (Calleary et al, 2009). Purulent epididymitis diagnosed by a combination of physical examination, ultrasound evaluation, and, occasionally, needle aspiration of the epididymis is an absolute indication for epididymectomy (Arbuliev et al, 2008). This is also the treatment of choice for epididymal abscesses and chronic infectious epididymitis that is unresponsive to antibiotic treatment. Diagnostic epididymal puncture and aspiration should not be performed in men with interest in future fertility, because this will result in epididymal obstruction. Epididymal malignancies are extremely rare, and 73% of nontransilluminating, solid epididymal masses are benign adenomatoid tumors (Beccia et al, 1976). Surgical extirpation should be considered for adenomatoid tumors, especially if there is any suspicion for malignancy (Alvarez et al, 2009). Partial/total epididymectomy can be approached scrotally through a median raphe or a unilateral transverse scrotal incision to deliver the testis. The vas deferens is identified, isolated, ligated, and divided. The testicular end of the vas deferens is then followed to the vasoepididymal junction. The tunica vaginalis is opened, and the plane of dissection between the epididymis and the testis is found in order to divide the epididymis from the testis. Great care should be taken to avoid injury to the spermatic cord and testicular artery. The efferent ducts superior to the testicular vasculature are ligated with an absorbable suture to complete the epididymectomy. The edges of the tunica vaginalis where the epididymis was excised are approximated with a running absorbable suture, which helps with hemostasis. The dartos fascia and skin are closed in layers with absorbable suture. In the case of partial epididymectomy, ligations are performed between the testis and epididymis with absorbable suture to excise the affected portion of the epididymis, while leaving the remainder attached to the testis with its vascular supply intact (Figs. 37-10 and 37-11). As discussed above, the majority of epididymal nontransilluminating masses are benign adenomatoid tumors. Fine needle aspiration of solid epididymal masses has been evaluated and found to be very accurate when compared with surgical pathology (Gupta et al, 2006). When malignancy is suspected, an inguinal approach should be used with clamping of the spermatic cord and delivery of the testis. The addition of testicular hypothermia by adding ice slush to the Chevassu maneuver (of clamping the vessels) was employed and found to salvage three out of five testicles with benign processes after clamping and parenchymal biopsy (Goldstein and Waterhouse, 1983). When malignancy is ruled out, the epididymal mass may be excised as for a spermatocelectomy.
Surgical Anatomy of the Scrotum
TESTIS
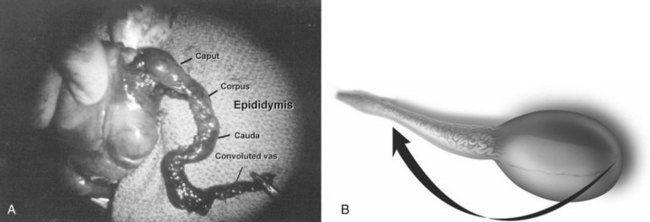
EPIDIDYMIS
VAS DEFERENS
Spread of Scrotal Infections and Postoperative Fluids Based on Scrotal Anatomy
Preoperative Preparation
Anesthetic Technique for Scrotal Surgery
Preoperative Preparation and the Use of Antibiotics in Scrotal Surgery
Surgery of the Scrotal Wall
Cyst Excision
Partial/Total Scrotectomy
Debridement of the Scrotal Wall in Fournier Gangrene
Vasectomy
Anesthetic Techniques for Vasectomy
Conventional Technique
“No-Scalpel” Technique
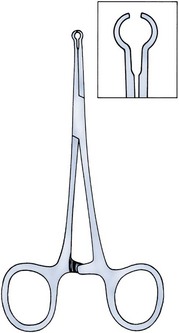
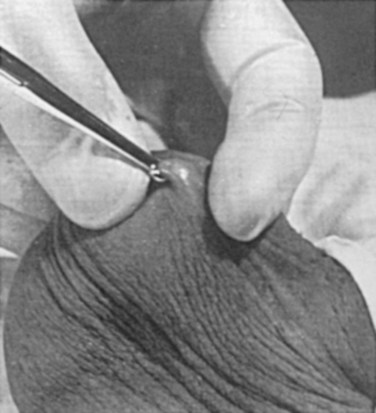
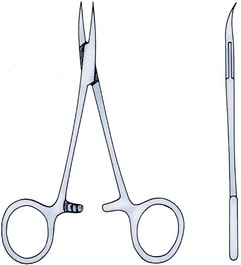
Methods of Vasal Obstruction/Male Sterilization
Performing Vasectomy to Make Microscopic Vasectomy Reversal Easier
Postoperative Care and Follow-up Semen Analysis
Complications: Local/Postoperative
Association of Vasectomy with Long-Term Systemic Disease
Antisperm Antibodies
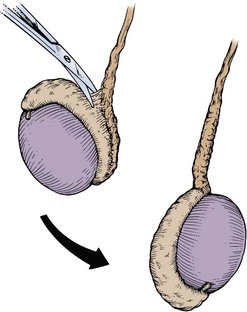
Spermatocelectomy/Surgery of the Epididymis
Surgical Indications
Partial/Total Epididymectomy
Excision of Epididymal Tumors
Surgery of the Scrotum and Seminal Vesicles