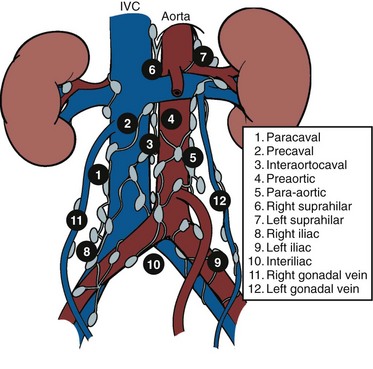
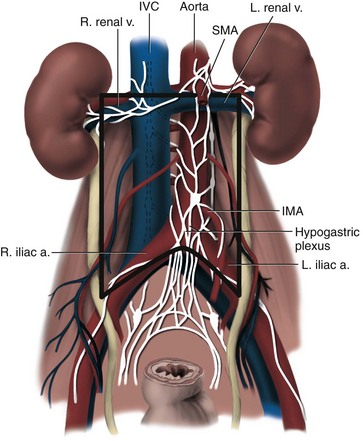
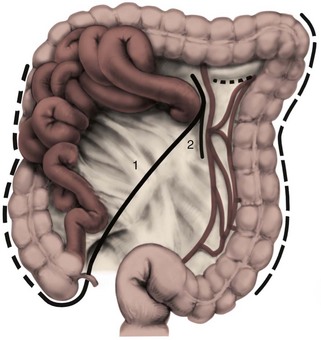
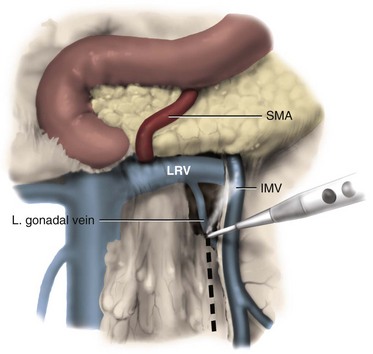
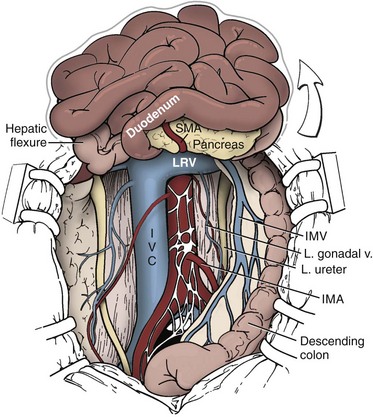
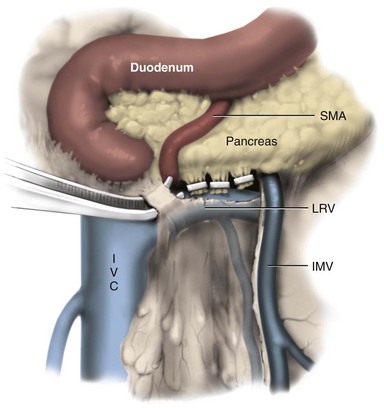
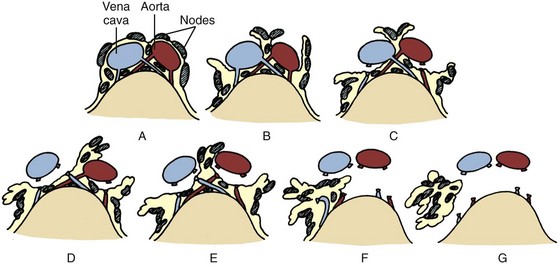
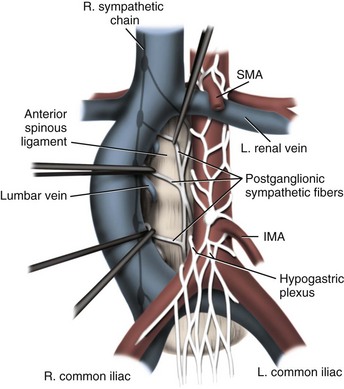
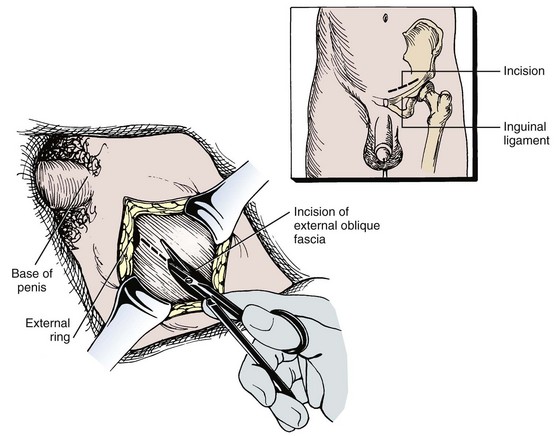
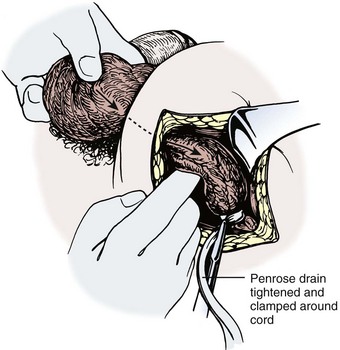
Joel Sheinfeld, MD, George J. Bosl, MD Each year, approximately 8000 new cases and almost 400 deaths due to testicular cancer are expected (Jemal et al, 2007). The incidence of germ cell tumors (GCTs), the most common solid tumor in men between the ages of 20 and 35 years, is rising both in the United States and Europe (Bergstrom et al, 1996; McKiernan et al, 1999). Given the dramatic improvements in survival rates from 60% to 65% in the 1960s to more than 90% in the 1990s, the management of testicular cancer has come to represent a model in the successful multidisciplinary approach to solid tumors (Einhorn et al, 1981). Although testicular cancer is highly curable, it requires appropriate management at all stages (Bosl et al, 2000). Both the cure rate and the morbidity are highly sensitive to nuances of management. The clinician and, more importantly, the patient pay a high price for inappropriate management. Surgery remains an integral part of the management of patients with GCTs, but the advent of effective chemotherapy and improvements in clinical staging, including sophisticated imaging modalities and reliable serum tumor markers, have altered its role over the past 30 years (Sheinfeld and Herr, 1998). Unfortunately, delays in the timely and accurate diagnosis of testicular cancer continue to be a significant problem. Moul (1994) noted a mean duration of symptoms of 26 weeks before diagnosis in a review of 4948 patients with testicular cancer, with both patient and physician factors contributing to this delay. Bosl and colleagues (1981) reported that the median interval of delay for patients with clinical stage I disease was 75 days compared with 101 and 134 days for patients with clinical stage II and III disease, respectively. Painless scrotal masses are often ignored, whereas testicular cancers presenting as scrotal pain are treated as epididymitis up to 18% to 30% of the time (Bosl et al, 1981; Prout et al, 1984). Almost 20% of patients present with signs or symptoms of metastatic disease, such as back or abdominal pain, weight loss, neck mass, and gynecomastia or breast tenderness (Bosl et al, 1981, 2000; Thornhill et al, 1987). Patients have undergone unnecessary mastectomy or laparotomy or prolonged therapy for back pain without considering the diagnosis of testicular cancer (Post and Belis, 1980; Moul and Moellman, 1992; Moul et al, 2000). Stephenson and associates (2004) reported on 40 patients with a midline retroperitoneal mass who underwent unnecessary laparotomy. At the time of laparotomy all patients had an abnormality on testicular examination or ultrasonography or an elevated serum tumor marker to suggest the diagnosis of GCT. The laparotomy contributed to therapeutic delay in a substantial number of patients and complicated their therapy. A radical orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the first step in the treatment of patients suspected of harboring a testicular neoplasm. This procedure provides histopathologic diagnosis and tumor (T) staging categorization, is associated with minimal morbidity and no mortality, and provides local control of the tumor in the vast majority of patients. The rare exceptions are usually due to tumor spillage, suboptimal orchiectomy, or transscrotal surgery (Whitmore et al, 1982). The procedure is performed under general, spinal, or local anesthesia on an outpatient basis. The patient is placed in the supine position with the scrotum prepped in the sterile field. A 5- to 7-cm oblique incision is made in the inguinal area along Langerhans skin lines approximately 2 cm above the pubic tubercle. This incision can be extended onto the upper scrotum to facilitate removal of large tumors. Camper and Scarpa fascia are incised to the level of the external oblique aponeurosis, which is then incised in the direction of its fibers to the level of the internal ring (Fig. 32–1). The ilioinguinal nerve is identified, dissected free of the cord, and preserved. The spermatic cord is isolated and occluded with either a noncrushing clamp or a 0.5-inch Penrose tourniquet at the level of the internal ring. The testis and its investing tunics are delivered into a carefully draped off field as gubernacular attachments are divided in the avascular plane. If a diagnostic biopsy or subtotal orchiectomy is planned, meticulous draping off is necessary before opening the tunica vaginalis and incising testicular parenchyma. Radical orchiectomy is completed by mobilizing the cord 1 to 2 cm inside the internal ring and individually ligating the vas deferens and the cord vessels between separate clamps (Fig. 32–2). The cord vessels are secured with silk ligatures, which can then be used to identify the stump if a retroperitoneal lymph node dissection (RPLND) is performed. The wound and scrotum are thoroughly irrigated and hemostasis is secured. A testicular prosthesis (soaked in antibiotic solution) can then be placed. The external oblique aponeurosis is closed with running 2-0 polypropylene suture. The Scarpa fascia is closed with absorbable sutures, and the skin is closed with either skin staples or a subcuticular suture. Compressive fluff dressings with a scrotal support minimize postoperative edema. (Adapted from Gottesman JE. In: Crawford ED, editor. Current genitourinary cancer surgery. Philadelphia: Lea & Febiger; 1990. p. 319.) (Adapted form Gottesman JE. In: Crawford ED, editor. Current genitourinary cancer surgery. Philadelphia: Lea & Febiger; 1990. p 319.) The most common complication of radical orchiectomy remains postoperative bleeding, which may occasionally result in either a scrotal or a retroperitoneal hematoma. Furthermore, significant retroperitoneal hematomas may delay further therapy or be misinterpreted as metastatic disease and result in unnecessary treatment (Bochner et al, 1995). Sayegh and associates (1966) reported that prior inguinal or scrotal surgery could alter the normal lymphatic drainage of the testis; consequently, many patients with scrotal violation have undergone extensive local surgery and/or adjuvant therapy to prevent an adverse outcome. Suboptimal approaches to testicular neoplasms including scrotal orchiectomy, transscrotal biopsy, or fine-needle aspiration are reported from 4% to 17% of the time (Capelouto et al, 1995; Leibovitch et al, 1995a). A recent meta-analysis of 206 cases of scrotal violation reported a local recurrence rate of 2.9% compared with 0.4% of patients treated by inguinal orchiectomy but no difference in systemic relapse or survival rates. There did not appear to be any advantage to adjuvant therapy (Capelouto et al, 1995). Others have reported an increased local recurrence rate in patients with scrotal contamination (Giguere et al, 1988) and an 11% presence of tumor in hemiscrotectomy specimens of patients with scrotal violation. Therefore the following recommendations for patients with scrotal violation seem prudent: A small subset of carefully selected patients with a solitary testis or bilateral testicular tumors, or a suspected benign lesion, may be candidates for “testis-sparing” surgery. The German Testicular Cancer Intergroup reported on 63 patients with median follow-up of 74 months and found only 4 local recurrences, all in patients with untreated intratubular germ cell neoplasia (Van der Schyff et al, 2000). Almost 90% of patients maintained normal testosterone levels. Favorable selection criteria include organ-confined disease with a mass less than 20 mm, negative postresection biopsies of the tumor bed, and absence of intratubular germ cell neoplasia (ITGCN) in the remaining testicular parenchyma. The procedure is performed under conditions of cold ischemia with great care to avoid tumor spillage or contamination. Biopsies of adjacent parenchyma are performed to identify ITGCN. The diagnosis of ITGCN on frozen section can be difficult due to artifact, and an experienced pathologist is particular helpful. The decision to perform completion orchiectomy in the presence of documented ITGCN is individualized based on preoperative discussion and the status of the contralateral testis. Patient compliance for rigorous follow-up must be assured, particularly because most patients with GCT have associated ITGCN. A subset of patients with documented advanced germ cell cancer that includes histologic verification from a metastatic site undergo systemic chemotherapy without orchiectomy. Rarely, postoperative complications after orchiectomy have resulted in delays initiating chemotherapy. The rationale for delayed orchiectomy after systemic chemotherapy is supported by data from a review of 160 patients in which 40 (25%) patients had viable cancer and 50 (31%) had teratoma in the resected testis (Simmonds et al, 1995). Staging evaluation must include thorough history and physical examination with particular attention to the contralateral testis. Serum tumor markers should be repeated immediately before RPLND and should include hCG, LDH, and AFP. Computed tomography (CT) of the chest, abdomen, and pelvis is the most efficient and cost-effective means of detecting metastatic disease. Magnetic resonance imaging (MRI) is usually reserved for the setting of major vascular involvement to assess patency of the inferior vena cava and renal vessels (Bosl et al, 2000). In 1997 the American Joint Committee on Cancer (AJCC) and Union Internationale Contre le Cancer (UICC) adopted a comprehensive staging system that now includes serum tumor markers (TNMS). Stage I refers to disease confined to the testis, stage II implies retroperitoneal metastases, and stage III disease indicates supradiaphragmatic or visceral metastases. In the TNMS system vascular or lymphatic invasion in the primary tumor are classified in the T2 category and serum tumor markers are included because of their independent prognostic significance (AJCC, 1998). Staging systems for testicular cancer are discussed in Chapter 31. Key Points Demographics The natural history of testicular cancer provides the basis for its evaluation and management, and, in turn, is favorably influenced by effective treatment (Whitmore et al, 1982). GCTs share several features that have contributed significantly to their successful management. These include (1) a germ cell origin, which is associated with responsiveness to irradiation (seminoma) and a number of chemotherapeutic agents, and with a potential for differentiation to histologically benign teratoma; (2) a rapid growth rate; (3) frequent production of specific tumor markers such as AFP and hCG; (4) usual occurrence in otherwise healthy young adults who can tolerate the necessary therapy; and (5) a predictable and systematic pattern of metastatic spread from the primary site to the retroperitoneal lymph nodes and subsequently to the lung and posterior mediastinum (Whitmore et al, 1982; Richie, 1992; Sheinfeld, 1994). Lymphatic spread is common to all forms of GCTs, although, in the case of choriocarcinoma, vascular dissemination is often a more common clinical feature. In 1899, Most was the first to accurately describe the lymphatic drainage of the testis (Most et al, 1899; Skinner and Leadbetter, 1971). Other anatomic studies in the early 20th century confirmed his findings and noted that the primary lymphatic drainage of the testis was to the area of its embryologic origin, that is, the retroperitoneal lymph nodes adjacent to the great vessels (Cuneo, 1901; Jamieson and Dobsin, 1910; Skinner and Leadbetter, 1971). Right-sided testicular drainage included the interaortocaval lymph nodes, followed by the precaval and paracaval nodes, whereas left-sided drainage included the left para-aortic and preaortic lymph nodes (Weinstein, 1999). Further anatomic studies and detailed mapping studies of RPLNDs have increased our understanding of the testicular lymphatic drainage and sharpened the focus of clinical staging and treatment by identifying the most common sites of metastatic disease (Busch and Sayegh, 1963; Chiappa et al, 1966; Sheinfeld, 1994). There are four to eight lymphatic vessels that accompany the spermatic vessels through the internal ring into the retroperitoneum. Although the majority of lymphatic channels continue to accompany the spermatic vessels to the point where those vessels cross the ureter and to their origin, some may drain directly into a lymph node on the anterior surface of the proximal third of the external iliac artery. At the point where the spermatic vessels cross ventral to the ureter, lymph channels fan out medially in relation to the aorta and inferior vena cava and drain into the retroperitoneal lymph node chain extending from approximately L5 to T11 (Weinstein, 1999). Contralateral lymphatic flow is often seen, particularly for right-sided lymphatics (Weinstein, 1999). Surgical mapping studies by Donohue and associates (1982), Weissbach and Boedefeld (1987), and Ray and colleagues (1974) divided the retroperitoneum into specific anatomic regions: right and left suprahilar, right paracaval, precaval, interaortocaval, preaortic, left para-aortic, right and left iliac, interiliac, and gonadal vessels (right or left) (Weinstein, 1999) (Fig. 32–3). The first echelon of lymph nodes draining the right testis is located in the interaortocaval area, followed by the precaval and preaortic nodes. The primary “landing zone” for left-sided tumors includes the para-aortic and preaortic lymph nodes, followed by the interaortocaval nodes (Donohue et al, 1982). More caudal deposits of metastatic disease usually reflect retrograde spread secondary to large volume disease and, rarely, aberrant drainage. Contralateral spread is more common with right-sided tumors, rare with left-sided tumors, and usually associated with large-volume disease (Sogani, 1991; Richie et al, 1992). Testicular tumors have the capacity to spread by direct extension and involve the epididymis and/or scrotum. The lymphatic drainage of the epididymis is to the external iliac chain, whereas that of the scrotum is to the inguinal lymph nodes (Osler, 1907; Weinstein, 1999). The rationale for treatment of the retroperitoneal lymph nodes in patients with testicular cancer is based on several factors. First, there is evidence that retroperitoneal lymph node spread is usually the first and often the only site of metastatic disease (Whitmore, 1979). This is supported by several clinical observations: (1) the survival rates in patients with retroperitoneal lymph node metastases treated by RPLND alone (Richie and Kantoff, 1991; Sheinfeld et al, 1999a; Stephenson et al, 2005); and (2) patients whose regional lymph nodes are found to be pathologically negative after adequate RPLND are usually cured by orchiectomy and the rare treatment failures in this group of patients are usually a result of pulmonary metastases and/or elevated serum tumor markers. Systemic relapse rates are declining and average approximately 10%, with disease-free survival rates ranging from 96% to 100% (Staubitz et al, 1973; Whitmore, 1979; Bredael et al, 1983; Stephenson et al, 2005). Second, although clinical staging continues to improve through refinements of radiologic imaging such as CT (Hilton et al, 1997), in 15% to 40% of patients their disease is clinically understaged, particularly in the retroperitoneum. This is supported by the 20% to 30% incidence of pathologic stage II disease in patients with clinical stage I disease, the approximately 25% relapse rate in the retroperitoneum on surveillance protocols (Lashley and Lowe, 1998), and the 20% incidence of teratoma and/or viable carcinoma in resected specimens of patients with subcentimeter lymph nodes on CT after chemotherapy (Fossa et al, 1989c; Toner et al, 1990; Sheinfeld and Bajorin, 1993; Oldenburg et al, 2003; Karellas et al, 2007). Third, untreated retroperitoneal lymph node metastases are usually fatal (Whitmore, 1979). Autopsy studies of patients dying with GCTs of the testis indicate that brain, liver, and bone metastases were late occurrences in the course of the disease and that most patients had concomitant and usually bulky retroperitoneal metastases (Johnson et al, 1976; Bredael et al, 1982). Interestingly, autopsy data did not reveal any significant differences in the site or frequency of metastasis between those treated before and after introduction of cisplatin-based chemotherapy (Bredael et al, 1982). Furthermore, the most common site of late recurrence of both teratoma and viable GCT is the retroperitoneum (Baniel et al, 1995c; Sharp et al, 2008). Late recurrences are usually chemorefractory, and survival rates are poor (Borge et al, 1988; Baniel et al, 1995; George et al, 2003; Carver and Sheinfeld, 2005; Sharp et al, 2008). Either a transabdominal or thoracoabdominal approach to the retroperitoneum for lymph node dissection may be used (Whitmore, 1962; Skinner and Leadbetter, 1971; Donohue, 1977). Initially, RPLND included bilateral suprahilar dissections as well as all the nodal tissue between both ureters down to the bifurcation of the common iliac arteries. Given the significant limitations in clinical staging at the time, and the absence of other effective therapeutic modalities, emphasis was necessarily placed on extensive dissection of all lymph nodes. Although its therapeutic efficacy was confirmed (Skinner, 1976; Donohue, 1977; Donohue et al, 1993), extensive suprahilar dissection can result in increased pancreatic, lymphatic, and renovascular complications. Several studies have confirmed that suprahilar metastases are rare in low-stage NSGCT, and, currently, suprahilar dissections are usually performed for residual hilar or suprahilar masses after cytoreductive chemotherapy for advanced-stage NSGCT (Ray et al, 1974; Donohue et al, 1982). In this setting the most common site of suprahilar disease is in the retrocrural space (Schmeller et al, 1981). In an effort to reduce surgical perioperative morbidity, bilateral infrahilar RPLND replaced the original suprahilar dissections (see Fig. 32–2) (Donohue et al, 1982, 1993). The procedure was associated with minimal morbidity and a mortality rate less than 1% (Baniel et al, 1994). The most consistent long-term morbidity of a standard bilateral RPLND has been the loss of antegrade ejaculation and, consequently, potential infertility, owing to damage of sympathetic nerve fibers (Lange et al, 1983, 1984; Jewett et al, 1988). Therefore patients are advised to complete sperm banking before the surgery. Prior to development of nerve-sparing techniques the incidence of this complication was associated with the extent of the retroperitoneal dissection (Lange et al, 1984; Jewett et al, 1988). The sympathetic chain, the paravertebral sympathetic ganglia, and postganglionic sympathetic fibers T2 to L4 and their convergence at the hypogastric plexus are most crucial in the preservation of antegrade ejaculation. Minimizing damage to these structures has resulted in higher rates of ejaculation (Lange et al, 1984; Sheinfeld and Herr, 1998). Two general approaches have been utilized to protect theses structures and include modified template RPLND and, more recently, nerve-sparing dissections, which can be applied to any template, including a bilateral template. Narayan and colleagues (1982) first reported that modification of surgical boundaries resulted in spontaneous return of ejaculation, with 25 of 55 patients recovering ejaculation at 3 years. Subsequently, a number of investigators have proposed a variety of modified RPLND templates for both right- and left-sided primary tumors with rates of return of ejaculation ranging from 51% to 88% (Fossa et al, 1984; Pizzocaro et al, 1985; Donohue et al, 1990; Richie, 1990). Successful preservation of ejaculation is higher for right-sided dissections compared with left-sided ones. In an effort to avoid disrupting the sympathetic nerves and the hypogastric plexus, all modified templates share several goals: (1) to thoroughly resect all ipsilateral lymph nodes between the level of the renal vessels and the bifurcation of the common iliac artery and (2) to minimize contralateral dissection, particularly below the level of the inferior mesenteric artery (Sheinfeld and Herr, 1998). Modified templates are based on mapping studies of retroperitoneal metastasis and aim to limit the extent of dissection in anatomic regions thought to be at decreased risk of metastatic spread. Unfortunately, mapping studies have several important limitations that contribute to an underestimation of the extent of retroperitoneal disease. Mapping studies often lack adequate postoperative follow-up, so the rate of recurrence outside the template region is unknown. Without appropriate follow-up, sample errors by the surgeon and/or the pathologist cannot be determined. For example, studies by Ray and associates (1974) and Donohue and coworkers (1982) did not report any follow-up data and the study by Weissbach and Boedefeld (1987) reported three retroperitoneal recurrences with a median follow-up of only 22 months in patients with pathologic stage I disease. This was a multicenter study that included 46 centers and 50 surgeons, thereby introducing significant surgical variability. Importantly, in the Weissbach and Boedefeld study it is not possible to accurately assess unresected metastatic sites in patients who subsequently received chemotherapy. In a recent analysis of 500 patients with clinical stage I and IIA disease who underwent primary RPLND at Memorial Sloan-Kettering Cancer Center (MSKCC), Eggener and associates (2007b) reported that depending on the template applied, 3% to 23% of patients with positive nodes will have extratemplate disease, including up to 11% with low-volume retroperitoneal disease. Similarly, Carver and colleagues (2007b) reported that, of 532 patients who underwent postchemotherapy RPLND (PC-RPLND) at MSKCC, up to 32% would have had disease outside the template region, depending on the limits of the modified templates. Both of these studies showed that the histology outside the template region was similar to that within the templates. This is an important observation, because chemoresistant teratomatous elements are present in approximately 30% of positive nodes in primary RPLND specimens and in approximately 40% of postchemotherapy specimens. Teratoma and malignant transformation are clearly overrepresented in patients who have experienced late relapse and repeat retroperitoneal surgery and arise from unresected disease (McKiernan et al, 2003; Sheinfeld, 2007; Sharp et al, 2008). These catastrophic sequelae highlight the potential danger of a reduced surgical field and overreliance on chemotherapy. Antegrade ejaculation requires the coordination of three separate events: (1) closure of the bladder neck, (2) seminal emission, and (3) ejaculation. The sympathetic fibers that mediate seminal emission originate primarily from the T12 to L3 levels of the thoracolumbar spinal cord. In the mid retroperitoneum after leaving the sympathetic trunk the fibers converge toward the midline and form the hypogastric plexus near the takeoff of the inferior mesenteric artery just above the aortic bifurcation. From the hypogastric plexus the sympathetic fibers travel via the pelvic plexus to innervate the seminal vesicles, vas deferens, prostate, and bladder neck (Fig. 32–4). Ejaculation is mediated by nerves originating at the sacral and lumbar spinal cord levels. Sympathetic fibers tighten the bladder neck, whereas pudendal somatic innervation from S2 to S4 causes relaxation of the external urethral sphincter and rhythmic contractions of the bulbourethral and perineal muscles (Lange et al, 1983, 1984; Sheinfeld, 1994; Sogani, 1991). The highest rates of preserved ejaculation are reported with nerve-sparing RPLND, in which the sympathetic chains, the postganglionic sympathetic fibers, and the hypogastric plexus are prospectively identified, meticulously dissected, and preserved (Jewett et al, 1988; Donohue et al, 1990). Nerve-sparing techniques can be utilized either in the primary or postchemotherapy setting and within standard bilateral or modified templates, depending on clinical and surgical circumstances; however, margins of resection should never be compromised in an attempt to maintain ejaculatory function (Bosl et al, 2000). The extraperitoneal thoracoabdominal approach to RPLND was originally described by Cooper and later refined and popularized by Skinner and their associates (Cooper et al, 1950; Skinner et al, 1982). The main advantages of this approach are to allow easier visualization and dissection of the suprahilar lymphatic tissues and less risk of postoperative small bowel obstruction. In addition, simultaneous thoracic procedures can be performed through the same incision. The patient is placed in the torqued position with the lower extremities and pelvis supine and the chest and upper extremities rotated medially. The operating table is hyperextended to allow exposure and retraction of the rib cage. The incision starts obliquely over the eighth or ninth rib and curves downward paramedian toward the pubic ramus. A subperiosteal rib resection is performed and the ipsilateral rectus muscle is divided. The peritoneum and contents are mobilized from the undersurface of the rectus sheath, and the diaphragm is divided and the pleural cavity is entered. The chest can be inspected and pulmonary or mediastinal procedures performed through the same incision. The retroperitoneum is exposed to the level of the contralateral ureter. Full bilateral RPLND can be performed as described for the transabdominal approach. In primary RPLND routine suprahilar dissection is not warranted, because the incidence of suprahilar or retrocrural disease is extremely low. In advanced disease, suprahilar dissection is directed by preoperative imaging studies and intraoperative palpation. The most common site of residual suprahilar disease is in the retrocrural space (Schmeller et al, 1981). The small bowel is reflected to the right, and an incision is made in the posterior peritoneum medial to the inferior mesenteric vein (Fig. 32–5). This incision is continued cephalad to the ligament of Treitz and is extended superiorly and medially to the duodenojejunal flexure, allowing for superior mobilization of the fourth portion of the duodenum and pancreas. The left leaf of the incised posterior peritoneum is further developed, with the colonic mesentery located anteriorly and the para-aortic retroperitoneal space posteriorly (Fig. 32–6). The proper plane of dissection is the avascular plane between the inferior mesenteric vein and the left gonadal vein. This maneuver further defines a thick condensation of fibrovascular tissue (ligament of Treitz) and several large lymphatic trunks, which should be divided between silk sutures, further mobilizing the tail of the pancreas. Alternatively, to gain adequate exposure in the area of the left renal hilum, particularly with large postchemotherapy masses, the inferior mesenteric vein can be doubly ligated and divided. The incision in the posterior parietal peritoneum is continued inferiorly along the medial aspect of the small bowel mesentery, lateral to the right gonadal vein and its branches, and extended around the cecum and up the right paracolic gutter to the foramen of Winslow. The duodenum is then kocherized, allowing cephalad reflection and exteriorization of the small bowel, cecum, and right colon onto the chest wall where they can be either placed in a Lahey bag or protected by moist laparotomy pads (Fig. 32–7). To facilitate retraction of the exteriorized viscera and minimize traction when self-retaining Gallagher and large Deaver retractors are placed, careful and thorough division of attachments between the undersurface of the duodenum and pancreas and the anterior surface of the left renal vein is necessary (Fig. 32–8). It is also important to ligate or clip the numerous lymphatic vessels in this area to minimize postoperative lymphatic complications. In placing self-retaining retractors great care must be taken in identifying the superior mesenteric artery to avoid vascular compromise of the small bowel. Periodic inspection of small bowel color or pulsation of the superior mesenteric artery is prudent. These maneuvers allow for excellent exposure of the retroperitoneal space from the level of the right and left suprahilar areas distally to the bifurcation of the right common iliac artery. Additional exposure of the distal left para-aortic and left parailiac space can be accomplished by further extending the incision in the left leaf of the parietal peritoneum inferiorly and, if necessary, sacrificing the inferior mesenteric artery. Alternatively, the left colon can be reflected medially by incising the left white line of Toldt and developing the plane between the colonic mesentery and the anterior surface of the Gerota fascia. Soft vessel loops are placed around the ureters as they are retracted laterally, and their medial aspect defines the lateral margins of resection. The ipsilateral gonadal vein is mobilized from its insertion in the inferior vena cava (right) or left renal vein (left) to the internal ring. It is important to encompass all the branches as well as the fibroalveolar and lymphatic tissue surrounding the gonadal vessels to avoid late paracolic recurrences from incomplete excision of the spermatic cord (Chang et al, 2002a). Attention is initially directed to the left renal vein, and the renal perivascular lymphatic tissue is mobilized inferiorly. The anterior surface of the aorta is thus exposed. Then adrenal, spermatic, and lumbar branches are tied with 3-0 silk and divided. The dissection along the anterior surface of the left renal vein continues to the right until the anterior surface of the inferior vena cava is encountered and the first anterior “split” is then performed. The “split and roll” technique is illustrated in Figure 32–9. The right gonadal vein is ligated at the vena cava. Lymphatic tissue can then be rolled off the inferior vena cava laterally and medially as the dissection proceeds inferiorly. Lumbar veins are dissected, doubly ligated with 3-0 silk, and divided. At this point, nerve-sparing techniques can be performed if clinically indicated (see later). Appropriate candidates for nerve-sparing techniques include patients with clinical stage I and low-volume stage II NSGCT undergoing primary RPLND as well as a carefully selected subset of patients undergoing postchemotherapy lymphadenectomy (Pettus et al, 2009). The most important aspect in performing nerve-sparing RPLND is the prospective identification and preservation of relevant sympathetic nerves, specifically (1) the sympathetic chains bilaterally, (2) the postganglionic sympathetic nerves arising from the sympathetic chains, and (3) the hypogastric plexus, which is the anastomosing network of nerve fibers anterior to the lower aorta (Fig. 32–10). The sympathetic chains run parallel to the great vessels on either side of the spine. On the left side the sympathetic chain is lateral and posterior to the lateral border of the aorta (see Fig. 32–10) and postganglionic fibers leave at an oblique angle transversing lymphatic tissue posterolateral to the aorta as they join the hypogastric plexus (Klein, 1995). On the right side the sympathetic chain lies posterior to the inferior vena cava and the postganglionic fibers emerge from the medial edge of the vena cava and course at an oblique angle anterior to the aorta to join the hypogastric plexus. Therefore it is important to note that an anterior “split” maneuver over the vena cava does not damage these fibers; however, dissection along the aorta before isolating and preserving these nerves individually results in their disruption (Klein, 1995). Several investigators have reported the use of intraoperative electrical stimulation of individual postganglionic sympathetic fibers to identify the most important nerves for antegrade emission (Recker and Tscholl, 1993; Kell and Jewett, 1999). Bladder neck closure and seminal emission can be documented endoscopically. In an effort to reduce the perioperative associated with a primary RPLND, several investigators have evaluated the role of laparoscopic RPLND (L-RPLND) in the management of clinical stage I and IIA and low-volume residual disease after chemotherapy. L-RPLND has been shown to be technically feasible in the hands of dedicated experts, although it involves a steep learning curve. In addition to a faster convalescence and more favorable cosmetic results, postoperative morbidity, perioperative blood loss, and length of hospital stay appear to be reduced compared with open surgery (Janetschek et al, 1996; Nielsen et al, 2007; Rassweiler et al, 2008), although no direct comparative studies have been done and the morbidity, blood loss, and hospital stay in open RPLND have dropped significantly during the same time period (Beck et al, 2008). However, the therapeutic efficacy of L-RPLND remains difficult to assess. First, the majority of patients with pathologic stage II disease receive chemotherapy after L-RPLND regardless of disease volume. A recent meta-analysis that included articles published since 2000 reported that 90% (126 of 140) of patients with positive nodes received postoperative chemotherapy. In spite of adjuvant chemotherapy the incidence of local and distant relapse rates were 1.4% (0.7 to 2.3) and 3.3% (1.8 to 4.6), respectively (Rassweiller et al, 2008). Second, it remains unclear whether L-RPLND has been performed with therapeutic intent. One group reports that L-RPLND is used for diagnostic purposes only and “patients with positive nodes are definitively treated with adjuvant chemotherapy” (Janetschek et al, 2000). A second group reports that the “dissection was limited if grossly positive nodes were encountered” and the total number of nodes resected was significantly different if the nodes were positive or negative (14 ± 2 and 25 ± 3, respectively) (Nelson et al, 1999). Disappointingly, a follow-up study that combined selected data from four of the most experienced groups in the United States continued to show a higher node count in pathologic stage I disease (median 22) compared with pathologic stage II disease (median 15.5). It is counterintuitive and inappropriate to perform a more extensive and thorough dissection in the absence of disease and a more limited one in the presence of retroperitoneal metastasis (Carver and Sheinfeld, 2005). The node counts in three contemporary series appear low, with means reported of 14.4 (range 5 to 25) (Campero et al, 2009); 14 (IQR 11 to 20) (Nicolai et al, 2009); and 14 (IQR 10 to 18) (Castillo et al, 2007). Third, there appear to be inconsistent and variable boundaries of dissection. Several laparoscopic surgeons recommend omitting dissection behind the great vessels and posterior to the lumbar vessels (Janetschek et al, 2000; Höltl et al, 2002) and advocate restricted templates even with pathologic stage II disease (Janetschek et al, 2000; Nielsen et al, 2007). The limitations of mapping studies and the incidence of extratemplate disease are discussed elsewhere in this chapter. Multifocality, contralateral, and extratemplate disease increase in pathologic stage II disease, and 20% to 30% will contain teratomatous elements, placing patients at risk for reoperative surgery, late relapse, and potentially inferior clinical outcomes (McKiernan et al, 2003; Eggener et al, 2007b). The indiscriminate use of chemotherapy is unnecessary in most cases of pN1 disease (Stephenson et al, 2005) and will not prevent relapse in cases of chemoresistant teratoma and a subset of viable cancer and increase long-term morbidity (Carver and Sheinfeld, 2005). Meinardi and colleagues (2000) reported a sevenfold increase in cardiovascular complications in men treated with cisplatin-based chemotherapy. On a positive note, a few highly specialized centers are now performing bilateral nerve-sparing RPLND, with preservation of antegrade ejaculation reported in 86% of patients. Follow-up is too short to evaluate the oncologic efficacy in pathologic stage II patients who did not receive chemotherapy (Steiner et al, 2008). Most worrisome are patterns of relapse not seen with open RPLND. Pizzocaro and associates (2007) reported on eight patients with clinical stage IA disease, two of whom with relapsing liver disease making them at poor risk by International Germ Cell Cancer Collaborative Group (IGCCCG) criteria. Although both were salvaged, the burden of therapy is necessarily increased, as is the potential risk of subsequent failure. There have also been reports of patients with liver metastasis and diffuse abdominal and pelvic carcinomatosis after L-RPLND (Bosl, 2008; Castillo, 2008). The presumptive mechanism for this unusual pattern of metastasis is the pneumoperitoneum and positive intra-abdominal pressure required to perform L-RPLND. The complication rate of L-RPLND after chemotherapy is higher than that of primary RPLND. Postchemotherapy desmoplastic reaction, as well as larger-volume residual disease requiring more extensive retroperitoneal dissection, increases the technical demands of the surgery. Nevertheless, investigators from Indiana University have reported a significant decrease in overall morbidity, major complications, and length of hospital stay (Mosharafa et al, 2004). Furthermore, for residual masses less than 2 cm, investigators from MSKCC reported a 4% major complication rate, including approximately a 2% incidence of bowel obstruction and a 1% to 2% incidence of chylous ascites (Patel et al, 2003). In the postchemotherapy setting, with the exception of the Austrian group who report minimal morbidity in carefully selected patients with low-volume residual masses, the conversion rate to open surgery is high and very significant complications have been reported. Rassweiler and coworkers (1996) converted seven of nine (78%) postchemotherapy patients with stage II disease from L-RPLND to open RPLND, and Palese and associates (2002) reported that two (28.5%) of seven patients required open conversion. Furthermore, in the Johns Hopkins experience of seven postchemotherapy patients with L-RPLND for residual masses with a mean size of 1.9 cm, the overall complication rate was 57%. Major complications included transection of the external iliac artery requiring bypass graft in a patient with a 1.5-cm residual mass, renal artery hematoma in a patient with mild adenopathy requiring aortorenal bypass, and a 4.5-cm residual mass in a third patient who suffered renal artery thrombosis requiring nephrectomy, subsequent duodenal perforation, necrotizing fascitis, and a cerebrovascular event with neurologic sequelae (Palese et al, 2002). The incidence of chylous ascites remains high (approximately 10%) after laparoscopic RPLND, in both the primary and the postchemotherapy setting (Wille et al, 2009). Consequently, in the postchemotherapy setting, L-RPLND cannot be endorsed as standard but rather as an operation to be evaluated in a formal prospective study by very experienced minimally invasive surgeons. Key Points RPLND In the United States and parts of Europe the conventional approach to patients with clinical stage I NSGCT has been bilateral RPLND, and it remains the standard against which all diagnostic and therapeutic alternatives must be judged (Whitmore, 1983; Hesketh et al, 1990; Chang and Sheinfeld, 2000). As noted earlier, RPLND (1) provides the most accurate N categorization of the retroperitoneal lymph nodes that are the first site of metastatic spread in approximately 90% of GCT and (2) is curative in the majority of patients with pathologic stage I (pNO) and low-volume retroperitoneal disease (pN1) and avoids the persistence of chemorefractory teratoma in the retroperitoneum (Sheinfeld et al, 2003). Relapses in the retroperitoneum are rare in the hands of experienced surgeons after properly performed RPLND. Primary RPLND is associated with negligible mortality and minimal and declining morbidity rates (Baniel et al, 1994; Stephenson and Sheinfeld, 2004; Stephenson et al, 2005; Beck et al, 2009). Nerve-sparing techniques should be considered the operation of choice, with reported antegrade ejaculation rates greater than 95% (Donohue et al, 1990). Surgical margins should never be compromised in an attempt to preserve ejaculation, and if positive nodes are noted or suspected at the time of RPLND a bilateral dissection is warranted, particularly for right-sided primary tumors, because contralateral crossover is more common than for left-sided primary tumors. The strategy of surveillance after orchiectomy was first reported by Peckham and colleagues in 1979. The rationale for surveillance protocols rests with the improved accuracy of clinical staging, the ability of cisplatin-based chemotherapy to cure early relapses, and the probability of surgical cure after radical orchiectomy alone and the possible infertility resulting from RPLND due to retrograde ejaculation (Sheinfeld, 1994; Bosl et al, 2000, 2005). Approximately 25% of patients with T1N0M0 disease and normal serum tumor markers experience relapse while on surveillance protocols (Hoskin et al, 1986; Swanson, 1993; Lashley et al, 1998; Sogani et al, 1998). The retroperitoneum is the most common site of relapse; the lungs or markers alone occur less frequently, and other visceral metastases are rare. Survival rates after therapy for surveillance failures range from 96% to 100% (Hoskin et al, 1986; Swanson, 1993; Lashley et al, 1998; Sogani et al, 1998). A number of factors predictive for retroperitoneal and/or systemic failure have been identified. The presence of lymphovascular invasion within the primary tumor (now included as T2), higher T stage (T2 to T4), that is, tumor involvement of the cord, capsule, or scrotum, and a high percentage of embryonal carcinoma are associated with a higher likelihood of relapse (Read et al, 1992; Gels et al, 1995; Nicolai et al, 1995; Heidenreich et al, 1998; Sogani et al, 1998). Approximately 50% of patients with T2 to T4 tumors experience relapse compared with 15% of patients with T1 tumors. One study from MSKCC noted that almost 80% of patients with clinical stage I and pure embryonal carcinoma who underwent RPLND had pathologic stage II disease (Pohar et al, 2003). Patient compliance cannot be overemphasized, because strict adherence to periodic follow-up evaluations is critical. Most relapses occur within the first 2 years and are rare after 5 years (Bosl et al, 2005). Of concern is the increased incidence of second malignancies reported in patients undergoing surveillance, presumably owing to the multiple CT scans required. Using the Surveillance Epidemiology and End Results (SEER) database, a retrospective study of, 334 patients demonstrated an odds ratio of 1.74 (1.02 to 2.95) for second malignancies associated with radiation exposure in patients on surveillance compared with those who underwent RPLND (Chamie et al, 2008). More recently, Tarin and colleagues (2009)
Management of the Primary Tumor
Radical Orchiectomy
Scrotal Violation
Partial Orchiectomy
Delayed Orchiectomy
Staging
Retroperitoneum and Germ Cell Tumors
Natural History, Patterns of Metastasis, Anatomic Considerations
Retroperitoneal Lymph Node Dissection
Evolution of Surgical Templates and Techniques
Modified Templates
Antegrade Ejaculation
Nerve-Sparing Techniques
Surgical Technique
Exposing the Retroperitoneum
Thoracoabdominal Approach
Transabdominal Approach
Setting Up the Dissection
Lymphadenectomy
Prospective Nerve-Sparing Techniques
Intraoperative Neurostimulation
Laparoscopic Retroperitoneal Lymph Node Dissection
Postchemotherapy Laparoscopic Retroperitoneal Lymph Node Dissection
Treatment Options for Low-Stage Germ Cell Tumors
Clinical Stage I NSGCT
Retroperitoneal Lymph Node Dissection
Surveillance
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of Testicular Tumors
1. In patients with low stage seminoma the radiation portals should be extended to include the ipsilateral groin and scrotum. This may result in an increased risk of azoospermia (Amelar et al, 1971).