Dye industry
Textiles
Printing
Rubber industry/Tyre manufacture
Steel industry
Cable manufacture
Leather industry
Petroleum
Shoemakers
Lorry drivers
Drill press workers
Rodent exterminators
Sewage workers
Hairdressers
In 1938, Hueper et al. [20] first demonstrated that the aromatic amine β(beta)-napthylamine could induce bladder cancer in dogs. Later, an epidemiological study by Case and Hosker [21] showed that exposure to α(alpha)-napthylamine, β(beta)-napthylamine, and benzidine were the main occupational factors in the development of bladder cancer. Some polycyclic aromatic hydrocarbons can also act as urinary tract carcinogens. Most carcinogens have a latent period of up to 20 years between exposure and the development of cancer.
Genetic Polymorphisms
Drug and carcinogen-metabolising enzymes are in part controlled by genetic polymorphism. Slow acetylation of N-acetyltransferase-2 (NAT2) [22, 23], rapid CYP1A2 activity [24], and glutathione S-transferase (GST) M1 null genotype [23] are associated with an increased risk of TCC. Approximately 20 % of Europeans are homozygous for a non-coding single nucleotide polymorphism (8q24.21) located close to the c-Myc oncogene [25].
Pathology
In Europe and North America, the majority of bladder cancer is of transitional cell origin. Transitional cell epithelium lines the renal pelvis, ureter, urinary bladder, and proximal urethra. Pure squamous cell carcinoma and adenocarcinoma account for less than 10 % of all bladder tumours, and small cell carcinoma, sarcoma, and malignant melanoma are rare. The term “superficial bladder cancer” is typically confined to TCC, as the other tumour types are usually invasive, and require radical or systemic therapy depending on type and stage.
The pathological classification and grading system for urothelial tumours was updated by the World Health Organisation (WHO) in 2004 [26], replacing the 1973 classification [27]. The previous three histological grades (grade 1, 2 and 3) were replaced by two (“low” and “high” grade). An overview of the two grading systems is shown in Fig. 31.1. At the time of writing, the 2004 system has not yet been universally adopted, and much of the evidence underpinning management of superficial tumours is based on trials in which patients were classified according to the old system.
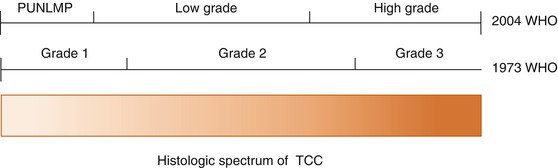

Fig. 31.1
WHO 1973 and 2004 grading systems (Reprinted from MacLennan et al. [81]. With permission from Elsevier)
The nomenclature relating to superficial bladder cancer can be confusing. The WHO 2004 system divides urothelial malignancies into Papillary Urothelial Neoplasms of Low Malignant Potential (PUNLMPs), Non-Invasive Papillary Urothelial Carcinoma (Low-grade), Non-invasive Papillary Urothelial Carcinoma (High-grade), and Invasive Urothelial Carcinoma. However, “invasive” in this usage refers to any tumour that invades the basement membrane, and as such includes tumours that invade the lamina propria but do not invade the detrusor muscle (stage T1, see section “Staging”). These tumours are, however, still considered “superficial” as they may be treated via endoscopic and intravesical means, without necessarily resorting to radical treatment. The presence of muscle invasion (stage T2 or greater) defines a tumour that is no longer “superficial”. It is this lack of clarity that has led to the proposal of the term “Non-Muscle Invasive Bladder Cancer” as opposed to “Superficial Bladder Cancer”.
Non-malignant Urothelial Tumours
The 2004 WHO classification system identifies several forms of non-malignant and pre-malignant urothelial tumour [26].
Urothelial hyperplasia is defined as markedly thickened mucosa without cytological atypia. It is seen in the mucosa adjacent to low-grade papillary tumours. There is no direct evidence of any premalignant potential, although it may be clonally related to nearby tumours.
Urothelial dysplasia by contrast does involve cytological atypia, but falls short of the changes seen in carcinoma-in-situ. It is challenging to diagnose and must be distinguished from reactive atypia, which is an inflammatory finding that may be seen in association with stones, infection or instrumentation. Urothelial dysplasia is a potentially premalignant finding. Clinical information on the outcome of de novo primary dysplasia is limited. In one study, 7 of 36 (19 %) patients with primary dysplasia developed carcinoma [28]. A later study showed that after a mean follow-up of 3.9 years, 4 (15 %) developed biopsy-proven cancer [29]. Another retrospective study of 15 patients found that 15 % with primary urothelial dysplasia and a mean follow-up of 4.8 years developed carcinoma in situ (CIS) [30]. The presence of urothelial dysplasia in association with papillary tumours increases the risk of tumour progression [31].
Non-malignant papillary tumours include urothelial papillomas and inverted papillomas. They are not pre-malignant and have a very low risk of local recurrence [26].
Malignant Urothelial Tumours
Papillary Urothelial Neoplasms of Low Malignant Potential (PUNLMPs)
This category of urothelial tumour in the 2004 WHO classification includes lesions that are similar in appearance to urothelial papillomas but show increased cellular proliferation exceeding the thickness of normal urothelium [26]. They would previously have represented the lower end of the spectrum of Grade 1 tumours using the 1973 WHO classification (Fig. 31.2).
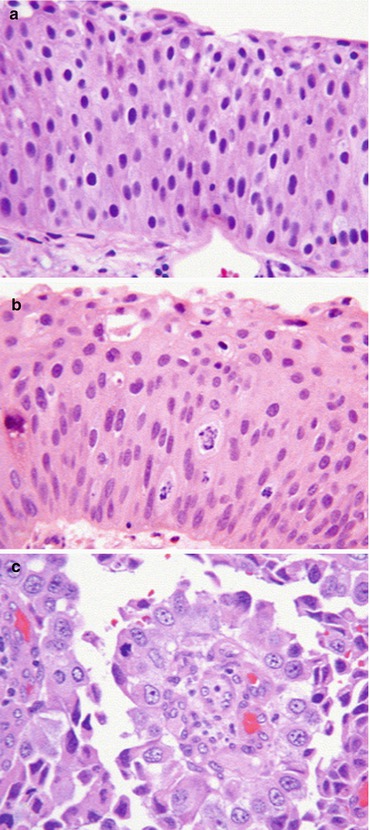

Fig. 31.2
Histological grades of urothelial tumour – PUNLMP, low-grade and high-grade. (a) Papillary urothelial neoplasm of low malignant potential (PUNLMP), formerly 1973 World Health Organization (WHO) grade 1 urothelial carcinoma. (b) Low-grade urothelial carcinoma, formerly 1973 WHO grade 2 urothelial carcinoma. (c) High-grade urothelial carcinoma, formerly 1973 WHO grade 3 urothelial carcinoma (Reprinted from MacLennan et al. [81]. With permission from Elsevier)
They are low-risk tumours in terms of recurrence and progression, with rates of 39 and 2.7 % respectively in a review of published series [32].
Non-invasive Papillary Urothelial Carcinoma – Low-Grade
This category includes the more aggressive grade 1 tumours and the less aggressive grade 2 tumours under the previous grading system. These tumours are unlikely to progress to invasive or metastatic disease, but recurrence is common, occurring in 48–71 % of patients [26].
Non-invasive Papillary Urothelial Carcinoma – High-grade
This category includes the more aggressive grade 2 tumours and all grade 3 tumours under the previous system, but is limited to those tumours that do not invade the lamina propria (stage Ta, see section “Staging”). These tumours have an increased risk of both progression to invasive disease and local recurrence.
Urothelial Carcinoma In-Situ (CIS)
Carcinoma in-situ is characterised macroscopically by a flat, velvety, erythematous patch within the bladder. It may occur as a solitary finding or in association with papillary or solid tumours. Microscopically, it consists of poorly differentiated malignant cells confined to the mucosa, without invasion of the basement membrane (Fig. 31.3).
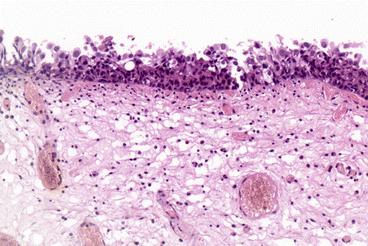

Fig. 31.3
Urothelial carcinoma in situ (Courtesy of Dr. John Dormer)
CIS represents high-grade disease by definition. Around 45 % of patients with primary CIS will progress to superficially invasive disease (stage T1, see section “Staging”) [33], with around 20–30 % going on to develop muscle invasion (stage T2).
When CIS is present in association with a non-muscle invasive papillary tumour, it acts as a significant risk factor for progression to muscle invasive disease. In patients with high-grade papillary disease invading the lamina propria (G3 T1), the presence of concomitant CIS increases the risk of progression to muscle invasion from 29 to 74 % at 5 years [34].
Invasive Urothelial Carcinoma
The WHO 2004 classification regards all tumours demonstrating lamina propria invasion (stage T1, see section “Staging”) as invasive urothelial carcinomas. These tumours are usually high-grade, and would have been considered grade 2 or grade 3 under the previous system. Only those tumours confined to the lamina propria without muscle invasion are regarded as superficial bladder cancers. Muscle invasive bladder cancer mandates radical and/or systemic treatment and is considered in a separate chapter.
High-grade T1 tumours are at considerable risk of both local recurrence and progression to muscle invasive disease. Around one-third of patients with this type of tumour will go on to die from bladder cancer, and a further third will require radical treatment due to the development of muscle invasion [35].
Micropapillary Variant
Micropapillary variant is an uncommon subtype of urothelial carcinoma that resembles papillary serous carcinoma of the ovary histologically [26] (Fig. 31.4). Its recognition is important as it may portend a poor prognosis in terms of the development of muscle invasion and lymph node and visceral metastases [36]. The amount of micropapillary change within the tumour specimen appears to be important, with higher percentages correlating with a poor response to intravesical treatment [36], and adverse outcomes [37]. Where tumours with extensive micropapillary change are encountered early radical cystectomy should be considered [36].
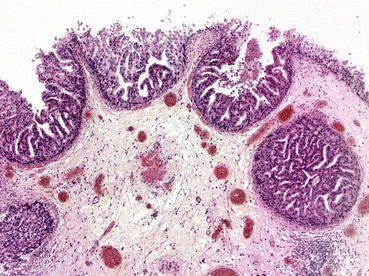

Fig. 31.4
Micropapillary variant (Courtesy of Dr. John Dormer)
Staging
The 2009 tumour, node, metastasis (TNM) classification is currently recommended for bladder cancer staging (Table 31.2) [38].
T | Primary tumour |
Tx | Primary tumour cannot be assessed |
T0 | No primary tumour |
Ta | Non-invasive papillary carcinoma |
Tis | Carcinoma in-situ |
T1 | Tumour invades sub-epithelial connective tissue |
T2 | Tumour invades muscle |
T2a | (Inner half) |
T2b | (Outer half) |
T3 | Tumour invades perivesical fat |
T3a | (Microscopically) |
T3b | (Macroscopically) |
T4 | Tumour invades adjacent structures |
T4a | (Prostate or cervix/vagina) |
T4b | (Abdominal or pelvic wall) |
N | Regional lymph nodes |
Nx | Nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Single involved lymph node in true pelvis |
N2 | Multiple involved lymph nodes in true pelvis |
N3 | Common iliac nodes involved |
M | Distant metastasis |
Mx | Distant metastasis cannot be assessed |
M0 | No distant metastases |
M1 | Distant metastases |
Histological categories Ta, T1, and Tis are regarded as superficial bladder cancers.
Sub-staging of T1 bladder tumours according to the depth of lamina propria invasion, using the muscularis mucosae as a reference point, has been proposed. T1a describes disease into the lamina propria but not invading the muscularis mucosae, T1b disease invades to the muscularis mucosae, and T1c beyond it. This sub-classification does not form part of the current TNM classification system, but has been shown to correlate with likelihood of progression to muscle invasion by Orsola and colleagues [39]. There was no difference in recurrence rates between the different sub-categories. Accurate T1 sub-staging represents a challenge for the pathologist, and was only possible in 87 % of cases in the study quoted above.
Pathogenesis
Molecular Pathogenesis of Low and High-Grade Tumours
Some molecular alterations are found in all grades and stages of TCC, such as chromosome 9 deletions, which appear to be an early genetic event. However, there are well-defined differences in the molecular changes seen in low-grade non-invasive tumours and high-grade tumours including those with lamina propria or muscle invasion, and carcinoma in-situ (CIS) [40].
Mutations of the fibroblast growth factor receptor gene (FGFR3) on chromosome 4p and the TP53 gene on chromosome 17p are almost mutually exclusive [41]. FGFR3 mutations are seen in PUNLMPs and low-grade non-invasive urothelial carcinoma, whereas TP53 mutations are seen in high-grade and/or invasive tumours. These differences at the molecular level underline the quite different behaviour of high-grade tumours in terms of their potential for invasion, metastasis and consequent mortality. These molecular alterations are outlined in Fig. 31.5.
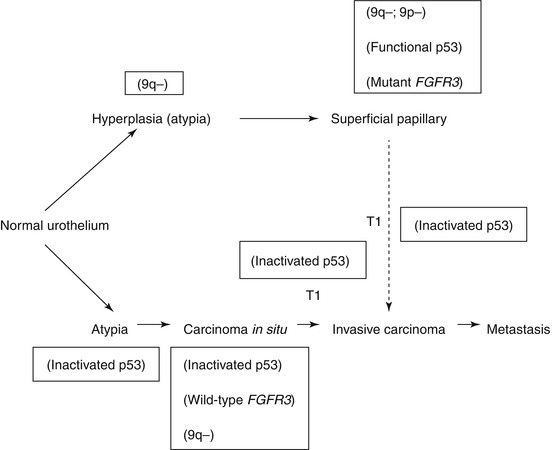

Fig. 31.5
Molecular pathogenesis of low- and high-grade tumours
By contrast, mutations of the FGFR3 and Ras genes in low-grade tumours are absolutely mutually exclusive, but Ras mutations appear to have no prognostic significance [41].
It is likely that in the future, molecular profiling using high throughput assessment of gene and protein expression will play an increasingly important role in stratifying superficial bladder tumours according to their likelihood of progression, and may be used to determine treatment or follow-up pathways.
Theories of Multifocality
Two principal theories have been proposed to explain the frequent multifocality of bladder tumours. The field-change, or oligoclonal, theory suggests that multifocal tumours arise due to individual transformation of areas of unstable or dysplastic urothelium into clonally unique tumours [42]. This theory is supported by the observation that tumours may recur months or years after treatment of the primary lesion.
There is some molecular evidence however, to suggest that in at least some cases recurrent or concurrent tumours may arise due to implantation or transepithelial spread of tumour cells, which are of a common clonal origin to the primary tumour [43].
The success of single instillations of intravesical chemotherapy after transurethral resection of superficial bladder tumours in preventing tumour recurrence has been attributed to prevention of implantation of tumour cells, which would lend further support to the theory of monoclonal implantation.
Clinical Presentation
The commonest presenting symptom of bladder cancer is painless visible haematuria, and approximately 85 % of patients present in this fashion. Some tumours, especially where CIS is present, may present with persistent irritative urinary symptoms, usually with concurrent haematuria (either visible or non-visible). Alternatively, tumours may be detected following investigation for asymptomatic non-visible haematuria detected during routine urine testing in primary care, or rarely with renal failure due to bilateral ureteric obstruction or symptoms of metastatic disease.
Investigations
Flexible Cystoscopy
Urine Cytology
Urine cytology was first described by Papanicolau and Marshall in 1945 [48]. When malignant cells are detected, it carries a high specificity for underlying urothelial malignancy (>95 %). However, its sensitivity is limited to 30–50 % mainly due to the fact that low-grade tumours are often not associated with malignant cytology. The accuracy of urine cytology is operator-dependent, with more accurate results from laboratories processing larger numbers of samples [49].
Urinary Biomarkers
There are a variety of urinary biomarkers available or being investigated for use in the detection and/or monitoring of urothelial cancer. In general, they possess greater sensitivity compared to cytology, at the expense of lower specificity. None, however, have sufficient sensitivity to obviate the need to perform cystoscopy [50].
Currently available point-of-care biomarker tests include Nuclear Matrix Protein 22 (NMP22) and Bladder Tumour Antigen (BTA Stat). Laboratory-based tests include BTA TRAK, ImmunoCyt and UroVysion.
Despite the demonstrably higher sensitivity of these tests compared to urinary cytology, their usefulness is limited by their reduced specificity. The concern regarding their use as a diagnostic adjunct in patients presenting with haematuria instead of cytology is that the increased false positive rate would lead to unnecessary anxiety and further investigations in patients with a positive biomarker assay but negative imaging and cystoscopy.
Other potential uses for these tests include for population-based screening, for which there is currently no evidence base, and as part of surveillance protocols for follow-up of patients after treatment for low- or intermediate-risk superficial bladder cancer (see section “Risk stratification”). It is proposed that the use of biomarker assays may reduce the frequency with which these patients require follow-up cystoscopy. It is this last application that looks most promising, but currently large prospective trials validating such protocols are lacking.
Imaging
Ultrasonography
Ultrasonography is often performed as part of the initial assessment of haematuria. It may demonstrate tumour within the bladder, although it will not detect small or flat lesions, including CIS, and it is dependent on the patient having a full bladder at the time of the test. It will also provide information about upper urinary tract obstruction, i.e. hydronephrosis, and may detect upper tract tumours, although its sensitivity for upper tract TCC is lower than IVU or CT urography.
CT Urography/Intravenous Urography
Computed tomography urography (CTU) has now largely replaced conventional intravenous urography (IVU) as the standard non-invasive imaging modality for detection of upper urinary tract tumours. Approximately 5 % of patients with bladder cancer will have synchronous or metachronous upper tract TCC. The incidence of synchronous upper urinary tract TCC is low (1.8 %), but higher in patients with trigonal disease (7.5 %) [51]. Patients with high-grade or multifocal tumours are at considerably higher risk of developing upper urinary tract TCC during follow-up [52], especially where CIS is present [53].
Cross-Sectional Imaging
Cross-sectional imaging with CT and/or Magnetic Resonance Imaging (MRI) for staging purposes is not required as part of the assessment of superficial bladder cancer, but is performed when muscle invasion is suspected or confirmed histologically after resection. MRI offers higher resolution for local staging, but, like CT, is unable to distinguish between post-resection inflammatory artefact and perivesical extension of tumour [54]. The two modalities are equivalent for assessing nodal involvement in the pelvis. For assessment of distant nodal disease and visceral or pulmonary metastases, CT is preferred.
Risk Stratification for Recurrence/Progression
TaT1 Tumours
The European Organisation for Research and Treatment of Cancer (EORTC) genitourinary (GU) group has devised a scoring system to predict the risk of recurrence and progression in the short and long term based on six clinicopathological variables (Table 31.3). These predictive variables were identified via a multivariate analysis of data from 2,596 patients with Ta or T1 bladder tumours, enrolled in 7 EORTC-GU trials [34]. The variables are weighted differently according to their influence on recurrence and progression to produce recurrence and progression scores (Table 31.4). These scores can then be used to stratify patients into low-, intermediate-, and high-risk categories for both recurrence and progression (Table 31.5). Criticisms of the EORTC tables include the possible bias inherent in using patients enrolled in trials, who may have more intensive follow-up than non-trial patients, and the fact that the cohort includes few patients with CIS, and few patients treated with BCG. Those patients that did receive BCG did not receive a maintenance regime and repeat transurethral resection (TUR) was not standard practice for high-grade T1 tumours. The Club Urológico Español de Tratamiento Oncológico (CUETO) group from Spain evaluated the EORTC tables in a cohort of 1,062 patients with superficial bladder cancer treated with BCG and found that they overestimated the risk of recurrence and progression in that group [55]. Despite these caveats, the EORTC tables have been validated in an external patient cohort with long-term follow-up [56], and are the risk stratification tool recommended by the European Association of Urology (EAU) [57].




Table 31.3
EORTC variables for assessing recurrence and progression
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







