INTRODUCTION
During the last decade, many new tools became part of the radiologist’s armamentarium to image the gastrointestinal (GI) system. Developments in computerized tomography (CT), magnetic resonance (MR) technology, and positron emission tomography (PET) made GI radiologists essential physicians in the diagnosis and evaluation of a vast array of diseases involving the abdomen and pelvis. Moreover, these major innovations mandated the replacement of some classic invasive diagnostic methods with noninvasive and efficient ones.
Multidetector-row CT (MDCT), initially introduced in 1998, has diffused into clinical imaging practice in a short time. Rapid volume coverage speed combined with thin image thickness allows creation of a volume data set. In addition to technical advances, such as shorter scanning times, multiplanar imaging, and improved ability to perform true multiphasic contrast-enhanced studies, advances in postacquisition data-processing techniques have made MDCT a powerful imaging tool in abdominal visceral imaging.
Compared with CT, magnetic resonance imaging (MRI) still plays a relatively minor role in the diagnosis and imaging workup of patients with abdominal diseases. However, technical improvements, such as the development of phased array multicoils, new contrast agents, and faster sequences, allow excellent contrast resolution images of the liver, pancreas, biliary tree, and even GI tract with an acceptable spatial and temporal resolution. Published results of MRI compared with CT and sonography have allowed, in a vast array of abdominal diseases, not only a more accurate delineation of the extent of disease, but also improvements in disease characterization. Furthermore, the evaluation of abdominal organs by means of contrast-enhanced dynamic MRI can be optimized with the use of MR angiography, which visualizes the vessels, and MR cholangiopancreatography (MRCP), which depicts the biliopancreatic ductal system. At present, this “all-in-one” approach is presumably the most cost-effective imaging technique in the evaluation of a vast array of liver function abnormalities, exocrine pancreatic diseases, and biliary disorders. Currently, the accepted indications for MRI of the GI tract include MR enterography (MRE), staging of rectal cancer and assessing the extent of perianal fistulae and anal sphincter tears. However, the value of MR colonography and MRI of appendicitis is rapidly emerging.
PET-CT is another rapidly evolving technique with increasing applications in GI diseases. The majority of investigations allow cancer staging and therapy monitoring, using the glucose analog 2-deoxy [18F] fluoro-D-glucose (FDG). Because tumor cells preferentially utilize glucose as a metabolic substrate, FDG-PET depicts areas of increased metabolism as “hot spots”; a CT scan performed in the same session allows linking these areas to morphologic abnormalities.
Coupled with these recent innovations in cross-sectional imaging, there has been a serious decline in the utilization of more routine diagnostic imaging methods used in the past to evaluate the GI system. Nowadays, fluoroscopic examinations of the small bowel and colon have very specific but limited indications. Nevertheless, some contrast examinations, such as barium swallow and upper GI studies, remain cost-effective investigations and continue to play an important role in the evaluation of the upper GI tract.
This chapter details the current imaging algorithms and key imaging features of various diseases involving the GI system, with special emphasis on new imaging techniques currently available in clinical practice.
GASTROINTESTINAL TRACT IMAGING
Although endoscopy may comprise the initial examination to evaluate the esophagus in many centers, fluoroscopic examination of the esophagus after contrast administration still has a high yield to detect clinically significant pathology and can complement endoscopy in several ways. Using a biphasic technique (single and double contrast), the esophagogram has proven invaluable in the evaluation of dysphagia, odynophagia, gastroesophageal reflux disease (GERD), and especially motility disorders; assessment of the integrity of the esophagus following local surgery or invasive procedures is another useful and common indication. Nowadays, CT and especially PET-CT are used increasingly in the evaluation of patients with esophageal neoplasms in order to stage the cancer locally and more distantly.
[PubMed: 18083069]
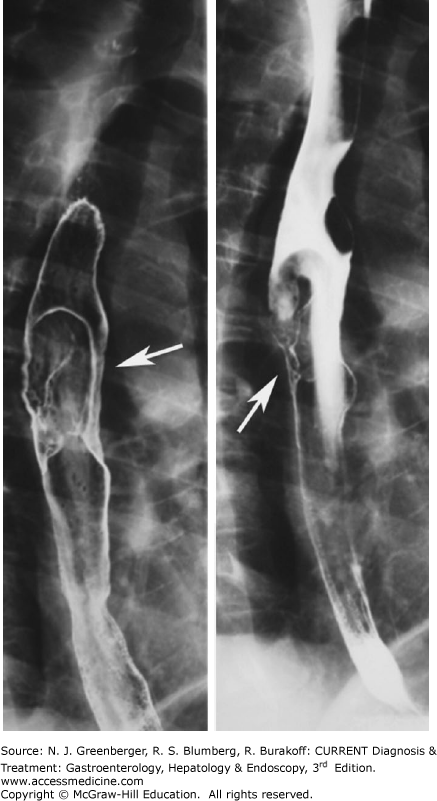
Esophageal carcinoma. Double-contrast esophagography has a sensitivity of greater than 95% in the detection of esophageal cancer. Approximately 70% of esophageal neoplasms are squamous cell carcinomas, and the remaining 30% are adenocarcinomas arising in Barrett esophagus. The appearance of esophageal cancers on barium studies is variable and has been described as infiltrating, polypoid, ulcerative, or varicoid (Figure 9–1). Fistula formation is seen in approximately 5–10% of cases. Nowadays, PET-CT imaging is increasingly used to detect nonregional lymphadenopathy or distant metastases (Plate 4) and may have a prognostic value.
[PubMed: 24794862]
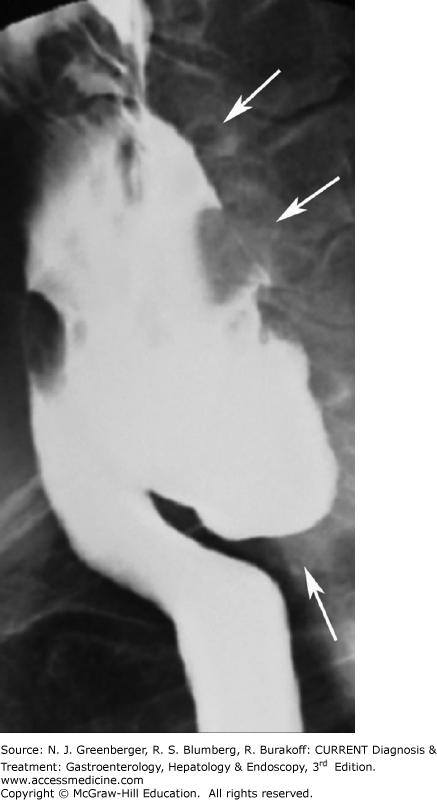
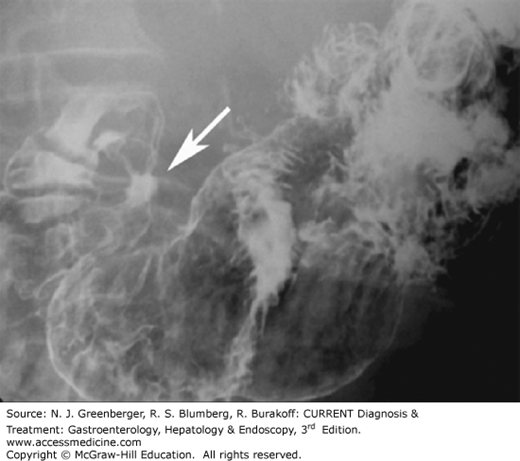
Zenker diverticulum (see Chapter 13) is an acquired mucosal herniation between the horizontal and oblique fibers of the cricopharyngeus muscle; it is more common in men and probably associated with GI reflux disease. On barium studies, a contrast-filled midline sac is seen posterior to the pharyngoesophageal junction (Figure 9–2). Below the opening of the sac, a prominent cricopharyngeal muscle is commonly identified. It is important for a Zenker diverticulum to be diagnosed on barium swallow because of an increased risk of perforation at endoscopy.
Gastroesophageal reflux disease. (See Chapter 11.) Barium studies have proved invaluable in patients with GERD to document the presence of a hiatal hernia, gastroesophageal reflux, and reflux esophagitis, and to detect complications, such as ulcerations, strictures, Barrett esophagus, and neoplasms. Barium studies, moreover, may be extremely useful in assessing symptomatic patients after antireflux surgery.
[PubMed: 24503359]
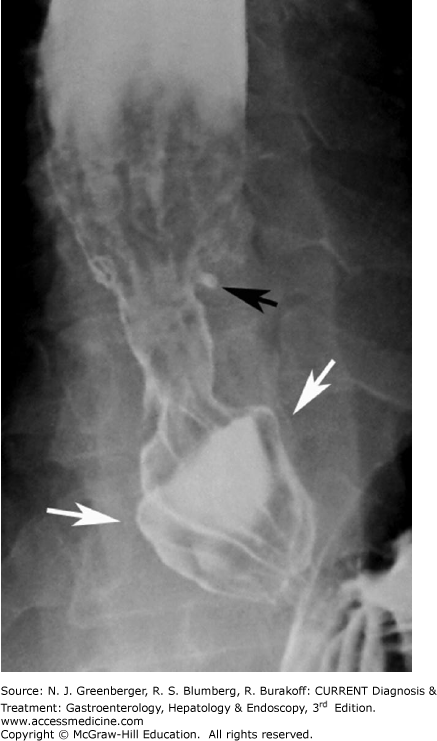
Early esophagitis in patients with GERD is manifested by a fine nodular or granular appearance with small well-defined radiolucencies caused by mucosal edema. In more advanced disease, contrast-filled ulcers may be present near the gastroesophageal junction on the posterior esophageal wall and peptic strictures may be detected in the distal esophagus above a hiatal hernia (Figure 9–3). The classic signs of Barrett esophagus include a high, midesophageal stricture, ulcer, or reticular pattern (see Chapter 12).
Infectious esophagitis is most frequently encountered in patients who are immunocompromised; among the possible infectious agents are Candida albicans, herpes simplex virus, cytomegalovirus (CMV), and HIV. Candida esophagitis (moniliasis) is typically manifested on barium studies as discrete, linear, plaque-like lesions separated by normal intervening mucosa, with a predilection for the upper and midesophagus. Herpes esophagitis shows the presence of small barium-filled ulcers on a normal background mucosa, while CMV and HIV esophagitis are manifested by giant, flat ulcerations that are typically several centimeters in length.
Achalasia can be categorized as primary when it is idiopathic and as secondary when it is caused by other conditions, such as tumors or Chagas disease (See Chapter 13). Patients with idiopathic achalasia are typically young and demonstrate lack of esophageal peristalsis and incomplete relaxation of the lower esophageal sphincter. On barium studies, the esophagus appears dilated, flaccid, and obstructed by a tapered, bird beak-like narrowing at the gastroesophageal junction. In patients with secondary achalasia, due to destruction of ganglion cells caused by miscellaneous processes, the length of the narrowed segment is often greater and may appear nodular or ulcerated.
Diffuse esophageal spasm (DES) is characterized by intermittent, abnormal esophageal peristalsis with presence of multiple, simultaneous nonperistaltic contractions. In approximately 15% of patients with DES, lumen-obliterating nonperistaltic contractions can be seen that compartmentalize the esophagus, producing the classic “corkscrew” esophagus on barium studies.
Upper endoscopy has largely replaced fluoroscopic studies as the primary investigation in patients with suspected gastric or duodenal pathology. Nevertheless, if endoscopy is contraindicated or unsuccessful, a well-performed upper GI study is the next sensitive diagnostic test. In addition to displaying the morphology of the stomach in patients following bariatric surgery, with suspected gastric volvulus, or gastric outlet obstruction, upper GI studies also readily detect mucosal and submucosal abnormalities that distort the gastric wall with high sensitivity. More recently, MDCT with neutral contrast material has been proposed as an excellent imaging test to evaluate the gastric wall, especially in the evaluation of gastric neoplasms. Other diagnostic methods utilized to diagnose and stage gastric neoplasms include ultrasonography, endoscopic ultrasound (EUS), MRI, and PET-CT scan.
[PubMed: 18096527]
Adenocarcinoma is by far the most common gastric malignancy and represents approximately 90% of all gastric malignancies. Diagnosis is reached through clinical suspicion and endoscopy. Gastric cancer staging is based on EUS, CT, and MRI. EUS may have a limited role in local staging, and, whenever possible, should be combined with other imaging modalities to optimize staging, while CT, and MRI in selected cases, can be more effective in staging advanced gastric cancer.
[PubMed: 23722535]
[PubMed: 25283742]
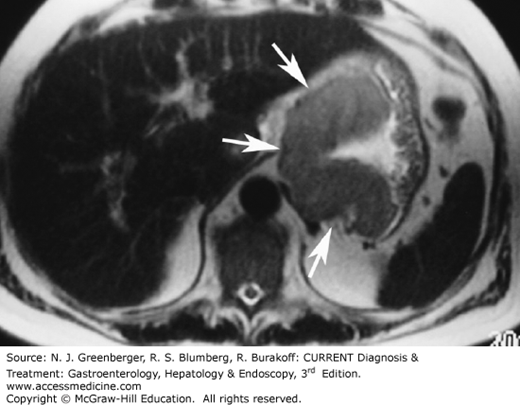
In particular, CT, currently the staging modality of choice, can identify the primary tumor, assess for local spread, and detect nodal involvement and distant metastases. On CT and MRI, if larger than 5 mm, gastric cancer may present as a focal enhancing soft tissue mass or as diffuse thickening of the gastric wall (Figure 9–4). Both CT and PET-CT have a reported sensitivity of 90% in detecting distant metastases. Classic features of gastric cancer include the presence of a lobulated or nodular mass with associated irregular ulceration (Figure 9–5). In scirrhous adenocarcinoma, the stomach demonstrates a long segment of circumferential narrowing and focal areas of mucosal nodularity.
Other gastric malignancies occur much less frequently and include lymphoma (<5%), GI stromal tumors (GISTs) (<2%), and malignant carcinoid (<1%). Gastric lymphoma typically appears as segmental or diffuse gastric wall thickening. In contrast to gastric cancer, lymphoma typically involves more than one region of the stomach, is unlikely to cause gastric outlet obstruction, and may be associated with lymphadenopathy below the renal hila. GIST can be benign or malignant, and 90% of them originate in the gastric fundus or body. Because of their extramucosal origin, upper GI series may be negative. Small masses appear as intra-mural hypervascular lesions on CT or MRI. Atypical GISTs, which show weak or negative KIT expression, may appear as heterogeneous masses containing cystic regions and soft tissue elements.
[PubMed: 16397744]
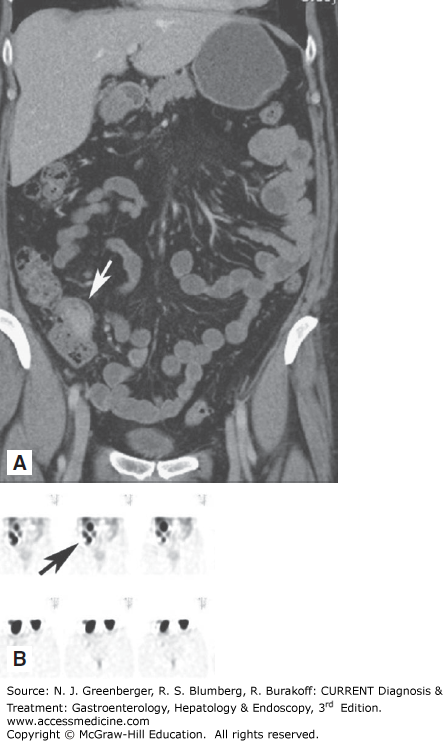
Larger stromal tumors tend to necrose, hemorrhage, or ulcerate (Figure 9–6). They typically are partially exophytic in location. Associated lymphadenopathy is uncommon, in contrast with gastric adenocarcinoma or lymphoma. PET-CT and diffusion-weighted imaging (DWI) MRI may be useful in the assessment of treatment response of GIST treated with targeted therapies, such as imatinib.
[PubMed: 23761549]
Peptic ulcer disease (see Chapter 15) is most commonly caused by infection by Helicobacter pylori or by nonsteroidal anti-inflammatory drugs (NSAIDs). Most duodenal ulcers are caused by H pylori, while most gastric ulcers are caused by NSAIDs. Erosion is an area of focal necrosis confined to the epithelium or lamina propria, whereas a true ulcer extends through the muscularis mucosa into the deeper layers of the gastric wall. On upper GI studies, a depressed lesion greater than several millimeters in depth is called an ulcer. When viewed en face, most peptic ulcers are manifested as round or ovoid collections of barium filling the ulcer crater with smooth, straight folds radiating to the ulcer’s edge (Figure 9–7). The presence of a Hampton line (a thin radiolucent line traversing the base of the crater due to undermining of the submucosa) is diagnostic of a benign gastric ulcer. CT does not detect most peptic ulcers because they affect only the superficial layers of the gastric wall. However, deep or perforated ulcers manifest as focal wall thickening with associated inflammatory changes in the adjacent soft tissues.
Gastritis, due to H pylori or other causes, typically appears on barium studies as thickened, scalloped folds that have a longitudinal or transverse orientation. Similarly, the most common CT finding in patients with gastritis is thickening of the gastric folds (Figure 9–8). In severe cases, the gastric wall appears stratified due to extensive submucosal edema and mucosal hyperenhancement. This striated appearance helps to distinguish gastritis from gastric cancers. H pylori gastritis, the most common infection in humans, is by far the most likely cause of focally or diffusely thickened folds, especially involving the antrum. Gastritis by a gas-producing organism (eg, Escherichia coli) may cause the classic appearance of emphysematous gastritis, a condition with a high mortality rate. Ménétrier disease typically causes massive enlargement of the rugal folds predominantly involving the gastric fundus and body with sparing of the antrum.
Traditionally, the small bowel has been studied noninvasively with fluoroscopic imaging techniques, such as small bowel follow-through (SBFT) or enteroclysis. The accuracy of SBFT and enteroclysis for detecting and characterizing small bowel abnormalities, however, varies widely among radiologists and institutions. Moreover, the inherent disadvantage of studying only the mucosa represents another limitation of enteroclysis and SBFT. CT enteroclysis is a successful alternative imaging method for a more detailed evaluation of bowel wall disease and for accurate diagnosis of extraluminal pathologies. With this technique, a nasogastric tube is fluoroscopically placed and oral contrast agent is infused subsequently; for most patients, however, the intubation remains a traumatizing experience. Today, imaging of the small bowel with noninvasive adequate distention of bowel loops is possible without fluoroscopically guided intubation. Recent studies have demonstrated that noninvasive, peroral CT enterography is an accurate and feasible technique for detecting active small bowel inflammation in patients with Crohn disease (CD). The use of a neutral oral contrast agent provides a better evaluation of the bowel wall due to the inherent contrast between the neutral bowel content and the enhancing bowel wall.
MRE is performed with increasing frequency due to the lack of ionizing radiation, which allows it to be used in young patients with Crohn disease who need repeated evaluation. MRE is also performed using oral and intravenous contrast material.
Remaining indications for plain film of the abdomen, SBFT, and enteroclysis in the evaluation of the small bowel include the detection and evaluation of adynamic ileus, small bowel perforation, small bowel obstruction, correct placement of jejunostomy tubes, and functional small bowel abnormalities.
CT enterography is preferably performed using an MDCT scanner after the oral administration of barium sulfate 0.1% w/v suspension. Patients drink 1350 mL of VoLumen (low-density barium sulphate preparation) prior to the scan. For specific indications, such as obscure GI bleeding, small bowel neoplasms, and chronic ischemia, a biphasic contrast-enhanced MDCT study is performed: images are acquired during the late arterial phase (40 seconds) or parenchymal phase (70 seconds) following intravenous administration of nonionic contrast material.
[PubMed: 16253662]
MRE is generally performed with the patient in prone position, with a 1.5 T scanner 60 minutes after oral administration of 1350–2000 mL of polyethilenglycol solution or biphasic contrast agent and IV injection of a spasmolytic (glucagon). A gadolinium-based contrast media is used to evaluate small bowel wall enhancement, and images are acquired before contrast media injection, during late arterial phase (40 seconds after contrast media injection) and delayed phase (70 seconds after contrast media injection).
In many institutions, SBFT is still the most common imaging technique used in the evaluation of suspected small bowel Crohn disease. Nevertheless, although SBFT can demonstrate early active mucosal disease, such as ulcers, it has limitations; most importantly, the radiologist must rely on secondary signs to evaluate the complete small bowel wall and mesentery.
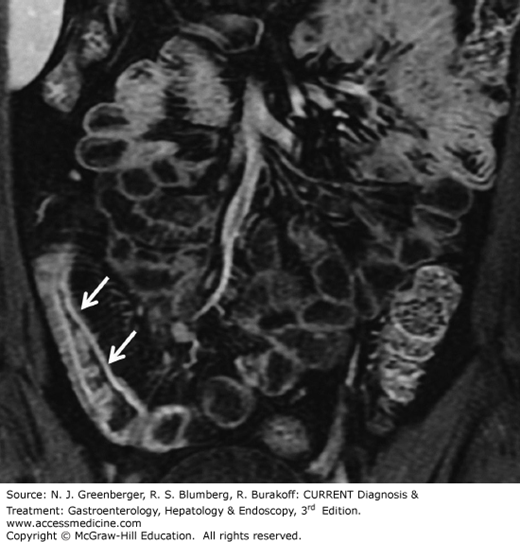
Although CT has traditionally been used to assess extraenteric complications of Crohn disease, including abscesses, fistulas, or obstruction, it has been shown that CT features of the small bowel wall itself, such as mural stratification, mucosal and mural hyperenhancement, edema in the mesenteric fat, and engorged ileal vasa recta, correlate with active inflammation (Figure 9–9). Furthermore, submucosal fat deposition and mural thickening without hyperenhancement or mural stratification typically correlate with fibrotic or quiescent disease. Large studies have evaluated the sensitivity and specificity of MDCT for detection of Crohn disease using SBFT as the gold standard; the sensitivity and specificity of MDCT for advanced disease were reported as 95% and 98%, respectively. In particular, adding multiplanar reconstructions to the axial data significantly improves the diagnostic confidence.
Recently, MRE has been introduced in clinical practice for the evaluation of Crohn disease.
While MRI and CT are equally accurate in assessing disease activity and bowel damage in CD, MRI is particularly valuable in assessing perianal lesions, such as perianal fistulae and perianal abscesses, and has higher soft tissue contrast resolution, when compared to CT. Furthermore, MRI is the desirable imaging modality due to lack of ionizing radiation. To monitor disease activity and to guide appropriate treatment, Crohn disease patients require multiple imaging examinations repeatedly.
[PubMed: 21701034]
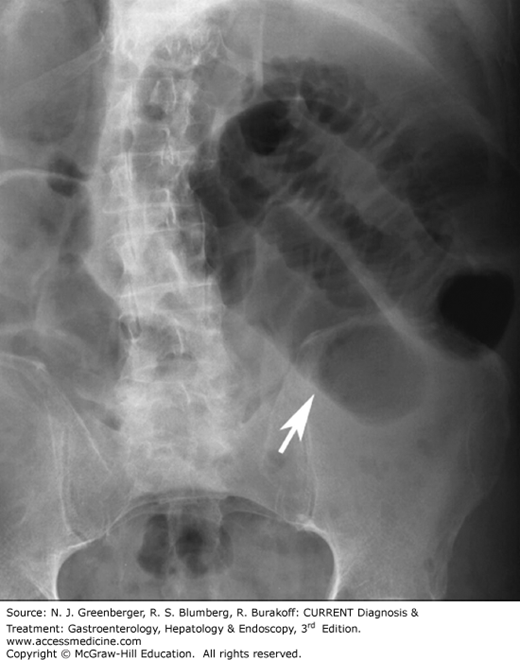
The early diagnosis of bowel obstruction is critical in preventing complications, particularly perforation and ischemia. The accuracy of conventional radiography in determining the presence of obstruction is 46–80% (Figure 9–10). Today, while abdominal radiography is often still the preferred initial radiologic examination, CT has gained favor because it can be effectively used to reveal the site, level, and cause of obstruction and it displays the signs of threatened bowel viability with high accuracy.
Adhesions are responsible for more than half of all small bowel obstructions, followed by hernias and extrinsic compression from neoplasms. Improvements in MDCT technology allow the transition point to be viewed from a variety of perspectives, increasing the diagnostic confidence once other causes have been appropriately excluded. Recently, Jaffe and colleagues reported a specificity of 87% and a sensitivity of 90% for diagnosing small bowel obstruction. MDCT is also excellent for detecting external hernias and for characterizing the bowel and mesentery in the hernia sac in patients with internal herniation.
[PubMed: 16293807]
Dual-phase MDCT can demonstrate changes in ischemic bowel segments accurately, is often helpful in determining the primary cause of ischemia, and can demonstrate important concomitant findings or complications (See Chapter 6). CT findings in acute bowel ischemia may consist of various morphologic changes, including homogeneous or heterogeneous hypoattenuating or hyperattenuating wall thickening, dilation, abnormal or absent wall enhancement, mesenteric stranding, vascular engorgement, ascites, pneumatosis, and portal venous gas (Figure 9–11). Dual-phase imaging of the abdomen is necessary during the arterial and the portal phases of enhancement to adequately opacify both the mesenteric arteries and veins. Three-dimensional (3D) imaging is crucial to visualize even the more distal branches of mesenteric arteries, which sometimes are not easily visualized on axial images. MDCT 3D imaging also can be used in the follow-up of patients after surgery, which typically includes bypass with grafts, whose patency is well documented by 3D imaging.
Diagnosis of small bowel tumors is still challenging because they are uncommon, usually produce nonspecific symptoms, and often are small. Therefore, MDCT, because of the thinner collimation, is achieving an even more important role in the detection and staging of small bowel tumors.
Recently MR enteroclysis has been proposed for patients with suspected small bowel tumors. In particular it has showed higher sensibility than CT in detecting mucosal lesions of the small bowel, and showed superior detection of segments with only superficial abnormalities.
[PubMed: 23524074]
[PubMed: 22821694]
Benign small bowel tumors are uncommon and are typically detected on SBFT CT or MR as incidental findings. The most common histotypes of these tumors are lipomas and leiomyomas. Lipomas are easily recognized by their fat attenuation values (–90 to –120 Hounsfield units [HU]). Leiomyomas are typically smooth, homogenous submucosal masses that enhance up to 1.5 times baseline following injection of intravenous contrast material.
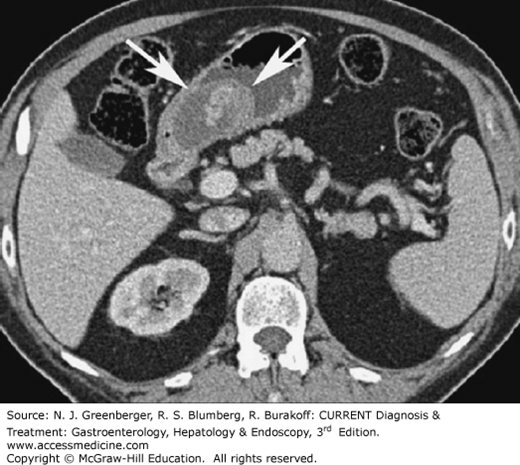
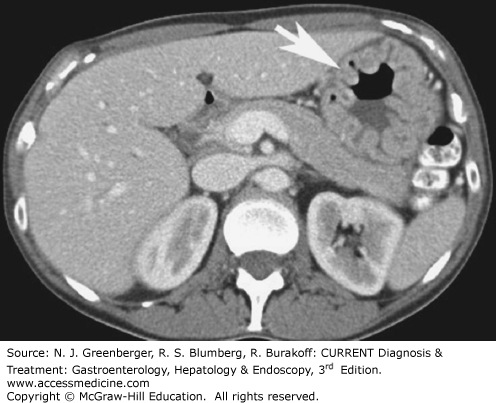
The most common primary malignant tumor of the small intestine is adenocarcinoma; it accounts for approximately 40% of all small bowel neoplasms. Most adenocarcinomas are located in the duodenum (Figure 9–12). On MDCT images, adenocarcinoma is typically visualized as a focal area of wall thickening that encases the lumen. The second most common primary small bowel neoplasm is carcinoid, representing approximately 25% of all primary small bowel tumors. Carcinoid tumors usually present as small enhancing nodules, which when bigger, may cause metastatic infiltration of the mesentery (Figure 9–13). On CT, this appears as an infiltrating mesenteric mass that contains calcifications in up to 70% of cases. Non-Hodgkin lymphoma represents approximately 10–15% of all small bowel neoplasms. The primary tumor often can be detected with small bowel contrast studies and CT; however, CT offers the advantage of simultaneously detecting adenopathy and the extraluminal extent of disease. Primary small bowel lymphomas often appear as a focally thickened, aneurysmally dilated loop. Celiac disease is a predisposing factor for small bowel lymphoma (Figure 9–14). GISTs are estimated to comprise 9% of all small bowel neoplasms. They typically appear as large, bulky masses. Central necrosis and ulceration are common. MDCT an MR imaging may be especially helpful in defining the exact site of origin and helping the surgeon plan for resection.
[PubMed: 18083648]
CrossRef
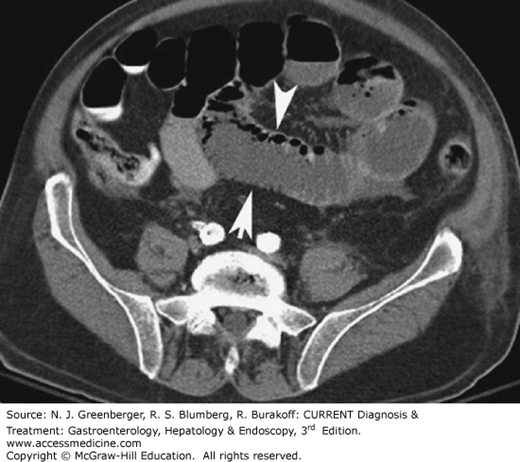
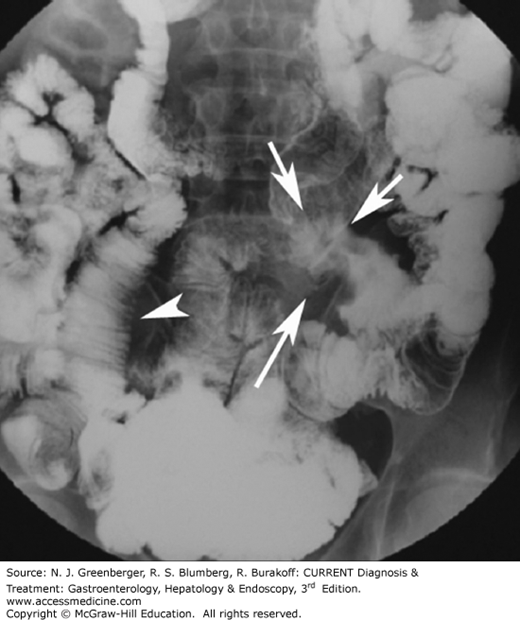
Several uncommon small bowel abnormalities, such as graft-versus-host disease, diverticulitis, radiation enteritis, intussusception, hernia, and volvulus, may be better visualized using multiplanar MDCT images than SBFT. On CT, the key to the diagnosis of radiation enteritis is the presence of abnormal findings only in the bowel loops localized to the area corresponding to the radiation port. Inguinal hernias can be either direct or indirect. Indirect hernias are the most common and occur predominantly in males. The peritoneal sac enters the inguinal canal and exits at the external ring. Because of their long course, indirect hernias can become incarcerated, leading to bowel obstruction and infarction. Direct hernias are less common and occur when the hernia enters the inguinal canal medial to the inferior epigastric vessels. On CT, finding of abdominal contents (small bowel, sigmoid, rectum, mesenteric fat) in the inguinal canal anterior to the femoral vessels is virtually diagnostic of an inguinal hernia (Figure 9–15).
Although sonography has proven to be a reliable technique to diagnose acute appendicitis in children and slim patients, it may be significantly limited in obese patients. Moreover, mild inflammatory changes of the appendix as well as retrocecal appendicitis may be missed if the cecum is air filled. In contrast, CT has an accuracy of up to 98% in diagnosing appendicitis and allows for diagnosis of even very mild and early stages of the disease. Furthermore, CT can often diagnose other pathologies that may clinically mimic appendicitis; CT is, therefore, able to decrease significantly the rate of negative appendectomies. Currently, MRI of appendicitis is increasingly used, with accuracy, sensitivity and specificity comparable with CT, and can be particularly useful in young or pregnant patients presenting with right lower quadrant pain and leukocytosis. In particular, T2-weighted sequences and gadolinium-enhanced sequences have proven particularly useful in children and adolescents.
[PubMed: 23481162]
[PubMed: 25341169]
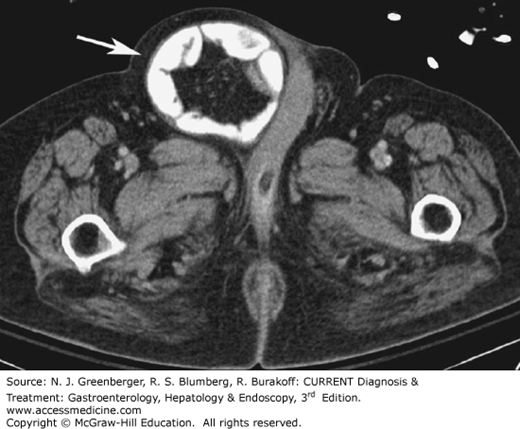
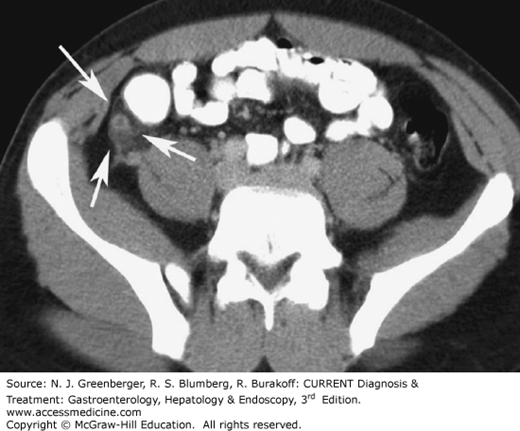
Typical CT findings of appendicitis are a thickened appendix with a diameter of more than 6 mm, with edematous or hyperwall, infiltration of the surrounding mesenteric fat, and, sometimes, inflammatory changes in the adjacent cecum (Figure 9–16). If an appendolith is present, along with pericecal inflammation, the scan is virtually diagnostic for appendicitis. The presence of inflammation in the surrounding mesenteric fat is reported to have a sensitivity of 100% and a specificity of 80%. Perforation is one of the serious complications that may occur in acute appendicitis, and CT can confirm this diagnosis. In cases with pronounced surrounding fluid collections, perforation should be considered. If extraluminal air can be identified in the peritoneal cavity or in the retroperitoneum or if the fluid collections have already taken on the appearance of a phlegmon or an abscess, perforation can be diagnosed with certainty. Lastly, in complicated cases, CT can also be used to diagnose pylephlebitis or pylephlebitic liver abscesses and for CT-guided abscess drainage in cases in which surgeons prefer to operate electively.
[PubMed: 15666398]
CT is almost universally accepted as the primary screening modality for the evaluation of patients suspected of having colonic disease. Key advantages of CT over other imaging modalities include the fact that CT not only allows accurate demonstration of the colonic wall but also outlines the pericolonic tissues and adjacent structures. CT can help assess infectious and inflammatory conditions, as well as facilitate diagnosis and staging of colonic neoplasms. The latter is typically performed using a dedicated CT method, known as CT colonography or virtual colonoscopy.
CT colonography has several advantages over optical colonoscopy: no sedation is needed, it is only minimally invasive, and the examination is less time consuming. However, there is still a need for bowel cleansing and insufflation of gas to expand the colon. Moreover, exposure to radiation is inherent to CT, and there is no possibility of biopsy or polypectomy or treatment. Failed optical colonoscopy or a medical contraindication for endoscopy is a good indication for CT colonography. There is still substantial debate about the usefulness of CT colonography as a general screening tool for colonic polyps and colorectal cancer. MR colonography is performed using contrast within the colonic lumen, resulting in increased and decreased signal intensity accordingly to the contrast used.
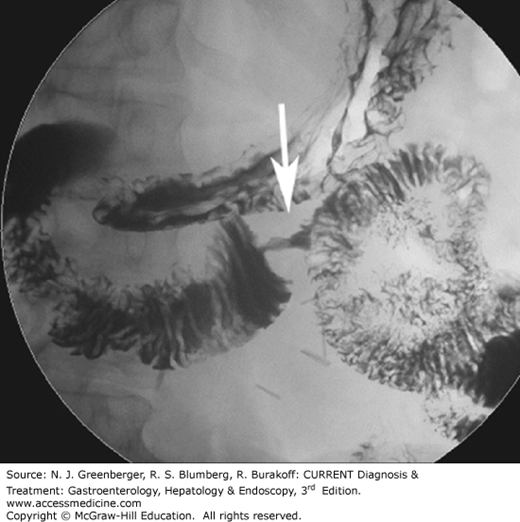
Remaining indications for single- and double-contrast barium enema are rapidly declining and are typically reserved for the postoperative patient, to detect strictures, fistula formation, or anastomotic leaks.
[PubMed: 25268304]
Optimal CT colonography technique requires careful cleansing and distention of the colon. Residual stool causes problems similar to those encountered with barium enema as it simulates polyps or masses. In theory, any preparation that results in a clean colon will suffice. Stool markers (mostly barium, eg, Tagitol) or iodine can be administered orally 24–48 hours before CT to improve differentiation of soft tissue intraluminal lesions and retained stool. This technique is called fecal tagging.
Three-dimensional endoluminal images are useful to confirm the presence of a lesion and to improve diagnostic confidence. Most investigators use a primary two-dimensional (2D) interpretation with so-called lumen tracking, starting from the rectum and following the course of the bowel from slice to slice to the cecum. Primary 3D endoluminal assessment of the colon (endoluminal “fly-through”) is less often performed as it is more time consuming and more susceptible to pitfalls. It is imperative that the 2D images are reviewed by using colon window settings (approximately W: 1600, C: –400). In addition, soft tissue windows must be used for further characterization of suspected lesions and when searching for extracolonic abnormalities.
As for CT colonography, careful cleansing and distention of the colon is required for MR. Colonic distension is gained through instillation of 1.5-2.5 L of warm water enema solution. Just before instillation of water, an antiperistaltic agent (scopolamine, glucagon) is administered. Sequences are obtained with patient in a supine position before and 75 seconds after IV injection of a gadolinium-based contrast media.
[PubMed: 19448111]
[PubMed: 24238128]
[PubMed: 20083594]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree