INTRODUCTION
Oropharyngeal and esophageal motility disorders can have a significant impact on patients’ quality of life. Diagnosis and management can be challenging because mechanical and functional problems may interact to cause patients’ symptoms.
Dysphagia (difficulty swallowing) must be distinguished from odynophagia (pain on swallowing, suggestive of a defect in mucosal integrity, eg, from trauma, irradiation, inflammation, or infection) and aphagia (inability to swallow, generally suggestive of acute obstruction). Symptoms that do not necessarily correlate with the immediate process of swallowing, such as rumination and globus sensation, should also be discerned.
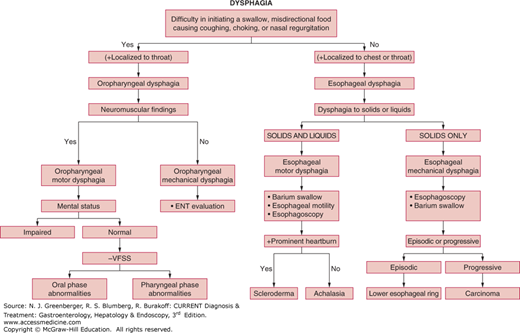
Dysphagia can considered arising from disorders in three anatomic phases of normal swallow (Table 13–1): (1) oral (also called preparatory) phase, (2) oropharyngeal phase (also called transfer dysphagia) involving the oropharynx, larynx, and upper esophageal sphincter (UES), and (3) esophageal phase, involving the esophageal body, lower esophageal sphincter (LES), and gastroesophageal junction (GEJ). The causes of dysphagia are many, and some may even overlap for both oropharyngeal and esophageal dysphagia (Figure 13–1). Specific entities are considered here.
Figure 13–1.
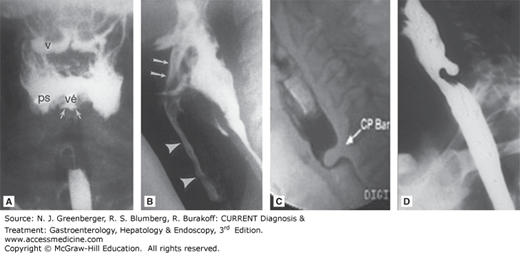
Radiologic appearance of oropharyngeal motility disorders. A. Frontal view of the pharynx demonstrates aspiration of retained bolus. Note that there is retention of contrast in the valleculae (v) and piriform sinuses (ps). No swallow is taking place, yet there is entry of contrast into the laryngeal vestibule (vé) and between the vocal folds and in the ventricle (arrows). B. A stop-frame print from a cinepharyngogram in the lateral position shows incomplete laryngeal closure during swallowing with laryngeal penetration (arrows) and aspiration (arrowheads) down into the trachea. The bolus is passing through the open cricopharyngeus into the cervical esophagus. Degenerative change is noted in the cervical spine. C. Cricopharyngeal bar. D. Zenker diverticulum. (Reproduced, with permission, from (A) Jones B (ed). Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy, 2nd ed. Springer-Verlag, 2003; (B) Jones B, Donner MW (eds). Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. Springer-Verlag, 1991.)
Oral phase Food enters mouth Chewing and mixture with saliva Bolus formation |
Oropharyngeal phase Tongue elevation and bolus delivery to pharynx Soft palate elevation, sealing nasopharynx Anterior and upward elevation of larynx and hyoid Posteriorly and downward closure by epiglottis Stopping respiration Shortening of pharynx |
Esophageal phase Quick relaxation of UES More prolonged relaxation of LES Bolus passage into esophagus Peristaltic contraction of esophagus Bolus enters stomach |
OROPHARYNGEAL MOTILITY DISORDERS
ESSENTIALS OF DIAGNOSIS
History of poor oral bolus preparation and control, difficulty in initiating a swallow, nasal and oral regurgitation, aspiration and coughing with swallowing, food sticking at the level of the throat.
Evidence of a generalized neuromuscular disorder.
Documentation by videofluoroscopic swallowing study (VFSS) or fiberoptic endoscopic evaluation of swallowing (FEES).
Many neuromuscular and structural disorders can cause oropharyngeal dysphagia (Table 13–2). Among these are cortical lesions, supranuclear, nuclear, and cranial nerve lesions, defects of neurotransmission at the motor end plates, muscular diseases, and obstructive lesions from luminal defects or from extrinsic compression.
Many patients with dysphagia are elderly and develop symptoms secondary to other disorders, especially diseases of the central nervous system such as strokes. In older patients, dysphagia is more often the result of head and neck injuries or cancers of the mouth or throat. Evidence of neurologic illnesses, nasal regurgitation and frequent coughing immediately upon swallowing, and poor oral coordination of bolus formation or dysphonia may help suggest the greater likelihood of oropharyngeal dysphagia over esophageal dysmotility. Diagnosis, assessment of severity, and optimal therapeutic intervention are generally guided by VFSS.
|
History and physical examination provide valuable information for identifying the etiology of the oropharyngeal dysphagia. Defects in different phases of oropharyngeal swallowing (see Table 13-1) should be identified by careful analysis of symptoms and signs.
Defects in the oral preparatory phase of swallowing manifest as chewing problems, oral stasis of food, inability to form a bolus, and coughing, choking, or aspiration pneumonia from regurgitation and aspiration. Poor dentition and inadequate saliva can aggravate underlying motility disorders. These symptoms occur during or immediately after the onset of swallowing and are usually more problematic with liquids than solids in causing symptoms of misdirection. However, patients after a stroke are prone to “silent aspiration,” that is, without cough or other outward signs of difficulty. Aspiration should be considered in mentally altered and debilitated patients with recurrent pneumonias, chronic congestion, and low-grade fever or leukocytosis, particularly if they have weak cough or voice, or a wet-hoarse or gurgly voice after swallows. The presence or absence of a gag reflex poorly distinguishes patients at higher risk for aspiration.
Generally, problems in the pharyngeal phase result in dysphagia, which the patient localizes to the throat. Often the patient makes repeated attempts to clear the throat of food or saliva. Halitosis can occur with large Zenker diverticulum, but could also be seen in esophageal causes such as advanced achalasia or long-term obstruction leading to decomposing food.
Abnormalities of the UES phase have no distinctive symptoms, but impaired UES opening further impairs pharyngeal transport and may aggravate the symptoms of pharyngeal stasis. On the other hand, a hypotonic UES may lead to esophagopharyngeal reflux and aspiration not related to swallowing.
Because many neuromuscular structures involved in swallowing are also involved in speech, dysarthria and dysphonia are common in these patients. Moreover, patients usually have evidence of neuromuscular defects in other parts of the body. Many patients with oropharyngeal dysphagia have impaired consciousness and cognitive functions that make evaluation difficult.
VFSS allows slow-motion replay of lateral and anteroposterior views of the oral and pharyngeal phases of swallow, which normally takes less than a second to complete. It is the study of choice for evaluating the severity and mechanism of oropharyngeal dysphagia and overt or silent aspiration (see Figure 13–1). VFSS, using different consistencies of the bolus and various head-positioning and glottis maneuvers, can also identify conditions to reduce aspiration. In comparison, barium swallow or an upper gastrointestinal series is generally not useful in evaluating oropharyngeal dysphagia. However, plain radiographs and computed tomography (CT) scan of the neck may reveal structural lesions such as tumors and cysts, and imaging studies may be obtained before upper endoscopy because pharyngeal and upper esophageal abnormalities such as diverticulas and malignant strictures can perforate in this poorly visualized region.
Because of the complex anatomy and radial asymmetry of the oral and pharyngeal passages, the speed of coordinated contractions, and common displacement of the catheter during the swallow, intraluminal manometry is not usually helpful and optimally requires catheters with solid-state transducers and possibly high resolution. It may be useful in the evaluation of upper esophageal function and resistance to flow across the UES, but these measures are variable with age, gender, and viscosity of bolus.
Regular upper endoscopy is generally not helpful in the evaluation of oropharyngeal dysphagia; however, videoendoscopy, available at some specialized centers, can provide information, particularly about structural causes of oropharyngeal dysphagia such as tumors and Zenker diverticulum.
Figure 13–2 is an algorithm outlining an approach to the patient with oropharyngeal dysphagia.
The major complications of oropharyngeal dysphagia are fatal pulmonary aspiration and pneumonia, malnutrition, and weight loss.
Evaluation and management by a deglutition team consisting of a deglutitionist (speech and swallow therapist), radiologist, gastroenterologist, otolaryngologist, and neurologist provide the best outcome in the care of these patients. Deglutitionists assess the risk of aspiration, type of food, and patient posture that is most likely to prevent aspiration and facilitate safe swallowing. Certain rehabilitative exercises to strengthen swallowing muscles may be helpful. Electrical stimulation of muscles is also being explored as newer avenues of therapy for oropharyngeal dysphagia. Investigations are performed to find the underlying cause of the disorder and appropriate therapy, if available, is initiated. If oral feeding cannot be undertaken safely, a percutaneous endoscopic gastrostomy (PEG) tube is placed by a gastroenterologist. The overall management of the patient, rather than focused treatment of the swallowing difficulty, is essential for effective management.
Prognosis depends on the underlying cause, compliance with therapy, and prevention of acute pulmonary complications. Patients with cerebrovascular accidents may regain their swallowing function after 6–8 weeks. Those with diseases such as myasthenia gravis, metabolic myopathies such as thyroid disorders, polymyositis, and Parkinson disease usually respond to appropriate treatment. Other patients, such as those with muscular dystrophy, amyotrophic lateral sclerosis, and multiple sclerosis, sometimes develop recurrent aspiration pneumonia that may prove fatal.
[PubMed: 15815202]
[JAMA and JAMA Network Journals Full Text]
UES dysfunction has been variably defined and described. Cricopharyngeal “achalasia” is a confused and often misused term since the diagnosis is rarely made by manometric or electromyographic evidence, but historically used upon radiographic evidence for impaired opening of the UES. Thus more precise terms should be used such as impaired UES relaxation, cricopharyngeal spasms, and cricopharyngeal bar. The clinical presentation of these entities comprising UES dysfunction is variable, but most patients complain of food sticking in the lower third of the neck. Patients may also experience heartburn, choking, and odynophagia, and less commonly dysphonia or globus sensation during swallows. The dysfunction is primary if confined to the cricopharyngeus muscle without neurologic or systemic cause, and secondary if produced by another disease process. Primary UES dysfunction, in turn, is subdivided into idiopathic and intrinsic myopathies (eg, polymyositis, inclusion body myositis, muscular dystrophy, and hypothyroidism). Secondary causes include amyotrophic lateral sclerosis, polio, oculopharyngeal dysphagia, stroke, and peripheral nerve disorders such as myasthenia gravis and diabetic neuropathy. Gastroesophageal reflux disorder (GERD) has also been suggested to cause cricopharyngeal spasm.
The diagnostic criteria for UES dysfunction remain controversial. The plain radiographic appearance is not reliable in making this diagnosis, and some experts advocate highly specialized radiologic (VFSS) and solid-state manometric probe studies. However, lack of data and standardization in manometric measurements (eg, in anteroposterior, lateral, or circumferential pressures), and the possibility of the manometric catheter eliciting cricopharyngeal spasms have thwarted their standard use. Many clinicians base their diagnosis simply on symptoms. Thus, the true incidence is unknown but may involve 5–25% of patients being evaluated for dysphagia.
A classic radiographic finding is a cricopharyngeal “bar,” or prominent projection on the posterior pharyngeal wall at the level of the larynx upon swallowing but with normal forward movement of the larynx on swallowing (see Figure 13–1C). This has been equated with UES “achalasia,” but manometric studies of patients with cricopharyngeal bars generally reveal normal UES pressure and relaxation, thus are not associated with impaired UES relaxation or UES spasms. Indeed, a transient cricopharyngeal bar is seen in up to 5% of individuals without dysphagia undergoing upper gastrointestinal studies and can be produced in normal individuals during a Valsalva maneuver. A persistent cricopharyngeal bar may be caused by fibrosis or frank myositis in the cricopharyngeus. Some cases have been reported with dermatomyositis or inclusion body myositis. However, muscle biopsies are not routinely taken.
UES dysfunction is poorly responsive to medical therapy including muscle relaxants. Cricopharyngeal myotomy is usually not helpful unless obstruction at the cricopharyngeus is demonstrated by videofluoroscopy in a severely symptomatic patient, such as those with significant aspiration or weight loss. Similarly, local injection of botulinum toxin A for cricopharyngeal achalasia is falling out of favor with recognition that the injection is rarely well localized to the affected muscles, leakage outside the cricopharyngeus may result in temporary dysphonia or aspiration, and effects appear to be short lived. However, a trial injection may be considered to aid in making the diagnosis, or for patients who are poor surgical candidates. These procedures are contraindicated in patients with cervical tumors, and relatively contraindicated in those with a fibrotic lesion after neck irradiation, or with a progressive neurologic disorder such as bulbar palsy. One exception appears to be patients with oculopharyngeal dysphagia, who appear to do well with surgical myotomy or with repeated dilations. Myotomy is also contraindicated in the presence of severe GERD because it may lead to pharyngeal and pulmonary aspiration. In patients with GERD, aggressive therapy with proton pump inhibitors (PPIs) is warranted in addition to management of the underlying disorder. The classic surgical approach is external cricopharyngeal myotomy.
Zenker diverticulum arises in the posterior wall of the hypopharynx, just above the cricopharyngeus muscle. The pathogenesis of Zenker diverticulum is not fully understood. It may form due to natural weakness of the pharynx (Killian triangle) associated with impaired opening of the cricopharyngeus muscle. Barium swallow or VFSS shows characteristic findings that allow easy diagnosis (see Figure 13–1D). With time, the diverticulum may become very large. Zenker diverticulum may retain food and secretions and classically leads to halitosis, delayed regurgitation, recurrent aspiration, and pneumonia. Dysphagia is usually due to compression of a food-filled diverticulum of the esophagus. Treatment is diverticulectomy with cricopharyngeal myotomy, and transoral approaches have also been introduced as an alternative and minimally invasive treatment.
Globus pharyngeus is a common functional disorder characterized by the persistent or intermittent nonpainful sensation of a lump or foreign body in the throat, but without any difficulty in swallowing or pain on swallowing. This disorder is more common in women than in men, and is often associated with an underlying psychiatric disorder or experienced during an emotional event. Some of these patients also have GERD, and ambulatory pH monitoring or empiric trial of acid suppression is recommended. The latter has been shown to improve symptoms in a third of the patients. Findings on barium swallow are generally normal, but may discern pharyngeal dysfunction or possible cricopharyngeal bar in patients with these symptoms. Esophageal manometry also may reveal achalasia in patients with these symptoms, even devoid of dysphagia. Results of upper endoscopy, when performed, are generally normal. However, one observational report describes improvement after ablation of cervical inlet patches. In most cases, treatment consists of reassurance. Patients with concurrent psychiatric disorders such as depression, panic, and somatization may benefit from tricyclic antidepressant therapy. Relaxation therapy has also been reported as helpful in refractory patients.
Three esophageal motility disorders commonly seen in clinical practice are esophageal motor dysphagia, GERD, and esophageal chest pain. In these disorders, symptoms result from dysfunction of one or more of the mechanisms necessary for normal esophageal function.
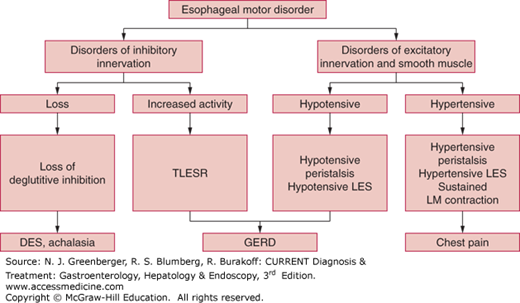
Esophageal motility disorders are classified, depending on the involvement of one or more of the three components of esophageal peristalsis, as disorders of inhibitory innervation, excitatory innervation, or smooth muscles (Figure 13–3).
The inhibitory innervation to the esophagus consists of vagal preganglionic neurons and the postganglionic neurons in the myenteric plexus, which release vasoactive intestinal peptide (VIP) and nitric oxide. The inhibitory pathway is responsible for relaxation of the LES and the gradient of peristaltic contraction in the esophageal body. Deficiency of inhibitory innervation results in achalasia and diffuse esophageal spasm. In achalasia, both the LES and esophageal body are affected, whereas in diffuse esophageal spasm, the esophageal body is primarily affected. Increased inhibitory nerve activity is responsible for so-called transient LES relaxation (TLESR).
The excitatory innervation consists of vagal preganglionic neurons and postganglionic neurons that release acetylcholine and substance P. The excitatory nerves contribute to basal LES hypertension, hypertensive contraction, and the force of peristaltic contraction. Deficiency of the excitatory nerves causes hypotensive LES and hypotensive peristaltic contractions. The esophageal body and LES consist of phasic and tonic muscles, respectively. Phasic muscles of the esophageal body contract during peristalsis, and tonic muscles of LES are responsible for tonic contraction. Muscle disorders may lead to hypotensive LES and hypotensive peristalsis.
In most conditions outlined below, a combination of imaging studies and intraluminal pressure measurements directs toward obtaining an accurate clinical diagnosis. Recent high-resolution esophageal pressure topography (EPT) obtained from high-resolution manometry has also allowed a hierarchical analysis of esophageal motility disorders.
[PubMed: 22248109]
[PubMed: 18364578]
ESSENTIALS OF DIAGNOSIS
Dysphagia to solids and liquids, localized to the chest or throat.
Associated symptom of chest pain and regurgitation.
Coughing and choking spells at night and unrelated to swallowing.
Symptoms of GERD.
Confirmation of abnormal motility by barium study and esophageal manometry.
Dysphagia must be distinguished from odynophagia (pain on swallowing); the latter suggests a breach in mucosal integrity by trauma, infection, and inflammation. The role of upper endoscopy is to rule out mucosal abnormalities such as strictures, webs, malignancies, infections, and eosinophilic esophagitis. Full-column barium swallow may reveal muscular rings, which are often missed on endoscopy. Manometric studies differentiate specific motility disorders.
Motor dysphagia in the thoracic esophagus occurs when deglutitive inhibition is lacking, due to loss of inhibitory nitrergic nerves, and peristaltic contractions become nonperistaltic; when the LES does not relax properly; or when the peristaltic contractions are weakened due to muscle weakness. Causes of esophageal motor dysphagia are listed in Table 13–3.
|