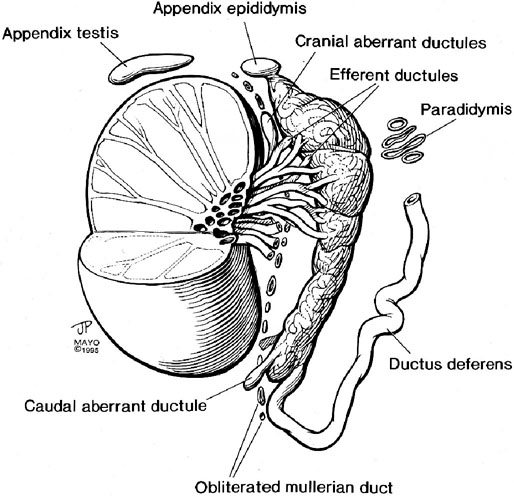
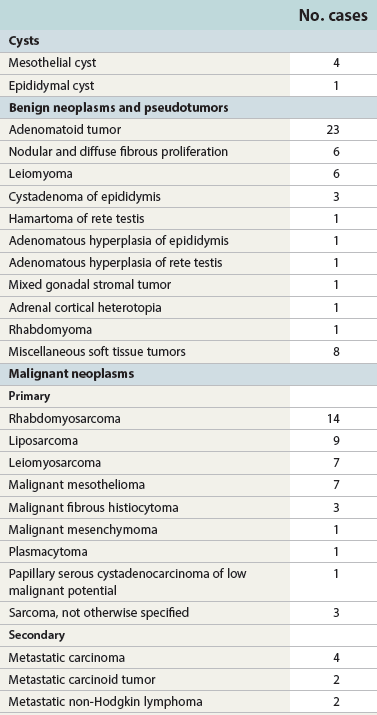
Chapter 14 The paratesticular region includes the testicular tunics, efferent ductules, epididymis, spermatic cord, and vas deferens. Most studies of paratesticular region pathology include the rete testis despite its intratesticular location.1 Numerous rare and interesting lesions arise in this region, including cysts, “celes,” inflammatory diseases, embryonic remnants, neoplasms, and neoplasm-like proliferations (Table 14-1). In children, one of the common neoplasms is paratesticular rhabdomyosarcoma. In adults, the most common pathologic conditions in order of frequency, excluding “celes,” are epididymitis, lipoma of the spermatic cord, adenomatoid tumor of the epididymis, and sarcoma of the spermatic cord.2 Table 14-1 Paratesticular tumors and cysts in the Canadian reference center for cancer pathology, 1949-1986 From Srigley JR, Hartwick RWH. Tumors and cysts of the paratesticular region [review]. Pathol Annu 1990:25(Part 2);51-108; with permission. The paratesticular region contains numerous anatomically complex epithelial and mesenchymal structures, often within embryonic remnants (Fig. 14-1). The rete testis of the mediastinum of the testis, the first element of the wolffian collecting system, connects the seminiferous tubules and efferent ductules. The embryology of the testis and its adnexa is described elsewhere; this chapter provides a brief summary of significant events in the development of paratesticular tissues. The testis and head of the epididymis arise from the genital ridge. The wolffian ducts—the male genital ducts—are paired tubes that are associated with the developing gonads and degenerating mesonephric tubules.3 The body and tail of the epididymis, the vas deferens, and the ejaculatory duct arise from the mesonephric tubules; other degenerating tubules often persist as embryonic remnants, including the appendix epididymis, paradidymis, and cranial and caudal aberrant ductules (see Fig. 14-1). The paired vasa deferentia connect to the ejaculatory ducts within the prostate, which have their outlets in the prostatic urethra adjacent to the müllerian tubercle. Blind diverticula of the distal vas deferens form the seminal vesicles. The müllerian duct, or paramesonephros, regresses in men, but may persist as embryonic remnants such as the appendix testis and prostatic utricle. The sac of the scrotum is divided by a partial median septum into two compartments, each of which contains a testis and epididymis and the lower portion of the spermatic cord. The scrotal wall consists of six layers, from the inside outward: the tunica vaginalis, the internal spermatic fascia, the cremasteric muscle, the external spermatic fascia, the dartos muscle, and the skin. The tunica vaginalis is a thin mesothelium-covered layer of the parietal peritoneum that also covers the white fibrous tunica albuginea of the testis and epididymis; it is initially in contact with the peritoneal cavity from which it arises, but becomes isolated with regression of the processus vaginalis. It is likely that a common stimulus, such as androgens, is required for obliteration of the processus vaginalis and epididymal development, a hypothesis supported by the common coexistence of epididymal anomalies and patency of the processus vaginalis.4 Development of the efferent ducts and ductus epididymis follows a biphasic pattern. Progressive development occurs from the fetal period to infants 2 to 4 months of age, but this development is transient and regresses during infancy. At childhood, definitive development is initiated and completed at puberty. These changes are probably related to the androgen dependence of the epididymis, the different stages of testicular maturation, and the steroidogenic activity of Leydig cells.5 The epididymis plays a critical role in maturation and viability of spermatozoa; SED1 facilitates epididymal cell adhesion, and its loss leads to breakdown of the epididymal epithelium and consequent development of spermatic granulomas.6 A variety of morphologic variations occur in the epididymal columnar cells and vas deferens, including cribriform hyperplasia (42% of patients),7 patchy or diffuse eosinophilic granular cell change (Paneth cell–like metaplasia) (8.3%),8 intranuclear eosinophilic inclusions,9 nuclear atypia with “monstrous” cells (14%),10 adenomatous hyperplasia, prostatic-type glands,11 epithelial luminal pitting,12 multiple diverticula in the cauda epididymis in the elderly,13 and accumulation of lipofuscin pigment.7 Agenesis and atresia of the testis, epididymis, and vas deferens result from failure of development of the genital ridge, often with anomalies of other wolffian derivatives and renal ectopia, agenesis, or dysplasia. Congenital absence of the vas deferens may be autosomal recessive, partial or complete, and unilateral or bilateral and is often associated with cystic fibrosis (see later discussion). Testicular biopsies in patients with congenital absence of the vas deferens reveal normal spermatogenesis or hypospermatogenesis in up to 45% of cases, and clinical investigation should include semen analysis, renal ultrasound, and genetic cystic fibrosis screening.7 Congenital unilateral absence of the vas deferens is more commonly associated with renal agenesis than bilateral absence (74% versus 12%, respectively).14 Congenital or developmental cysts of the epididymis are extremely rare and may be associated with intrauterine exposure to diethylstilbestrol.15 The cysts are usually solitary, but may be multiple and bilateral. Ectopic epididymis may be found anterior to the testis, in the retroperitoneum, and within the kidney. Epididymal abnormalities are commonly associated with ectopic or cryptorchid testes (72% of cases), ranging from simple elongation of the epididymis (33%) to more complex changes such as complete disruption (39%).16 Splenogonadal fusion is a rare congenital anomaly in which fusion of the splenic and gonadal anlage occurs.17 Approximately 100 cases have been reported, usually on the left side (98%) in men (95%). Patients may present with a nontender scrotal mass or intestinal obstruction, but most cases are discovered incidentally at autopsy or surgery for cryptorchidism or inguinal hernia. Approximately 57% are associated with other congenital anomalies, including peromelia, micrognathia, and cardiac anomalies. Hepatogonadal fusion has also been reported.18 Adrenal cortical tissue may be present anywhere along the route of descent of the testis from the abdomen to the scrotum (Fig. 14-2).19 It is usually an incidental finding at inguinal herniorrhaphy or epididymo-orchiectomy, present in 1% to 3% of children undergoing such operations.7 Adrenal cortical tissue has been identified in the inguinal hernia sac, spermatic cord (see Fig. 14-2), epididymis, and rete testis. It may manifest as a palpable tumor and appears as small, round to oval, yellow-orange nodules, 1 to 5 mm in diameter, usually near the inguinal ring. The lesions almost always consist of adrenal cortical tissue resembling zona glomerulosa and fasciculata. Rarely, they contain medullary tissue. Involution during childhood is the rule, but exceptional cases persist and become functional, rarely harboring neoplasms or developing into tumors in adrenogenital syndrome and Nelson syndrome. Removal of functional rests may result in adrenal insufficiency. Ectopic renal tissue has been observed, rarely, in the scrotum, consisting of tubules and immature glomeruli. Numerous embryonic remnants are found in the paratesticular area, including the appendix testis (hydatid of Morgagni), appendix epididymis, paradidymis, and vasa aberrantia. Precise classification of cystic remnants may be challenging.7 The appendix testis is present on more than 90% of testes at autopsy; ultrasound examination found an incidence of 44%.20 This structure is located at the superior pole of the testis adjacent to the epididymis. Grossly, it varies from 2 to 4 mm, appearing as a polypoid or sessile nodular excrescence. Microscopically, it contains a fibrovascular core of loose connective tissue covered by simple cuboidal or low columnar müllerian-type epithelium that is in continuity with the tunica vaginalis at the base. The fibrovascular core may contain tubular inclusions lined by similar cuboidal epithelium. Torsion of the appendix testis may be painful, may mimic testicular torsion, and is the most common cause of acute scrotum in children.21 The severity of the acute inflammatory cell infiltrate is predictive of the longer duration of symptoms and presence of clinical evidence of torsion of the testicular appendages.22 No other association was detected between the pattern or degree of acute inflammatory cell infiltrate or any other clinicopathologic variable that might indicate pyogenic infection. No bacteria or fungal elements were identified. Marked lymphatic dilation may be the only histologic finding to indicate the presence of early torsion in cases of scrotal pain secondary to torsion of the appendix testis. The appendix epididymis is present on approximately 35% of testicles examined at autopsy; ultrasound examination found an incidence of 18%.20 Grossly, it is a pedunculated spherical cystic or elongate structure arising from the anterosuperior pole of the head of the epididymis. Microscopically, it is lined by cuboidal to low columnar epithelium that may be ciliated and show secretory activity. The wall consists of loose connective tissue and is covered on its outer surface by flattened mesothelial cells that are continuous with the visceral tunica vaginalis. The appendix epididymis may become dilated by serous fluid and when enlarged may mimic a tumor. Torsion may occur, sometimes in cryptorchidism. This wolffian duct embryonic remnant consists of clusters of tubules lined by cuboidal to low columnar epithelium within the connective tissue of the spermatic cord, superior to the head of the epididymis.23 These wolffian duct remnants appear as clusters of tubules that are histologically similar to the paradidymis. They arise within the groove between the testis and epididymis. Torsion of the vas aberrans is rare.24 Herniorrhaphy in children is surgically challenging, particularly in the commonly strangulated hernia sac, accounting for the vulnerability of the epididymis and vas deferens that may be inadvertently transected during the procedure. This problem is compounded by the diagnostic difficulty in classifying glandular inclusions in hernia sacs. It is an especially challenging problem in young children because of the ambiguity of distinguishing features among the structures and embryonic remnants prior to puberty. Benign glandular inclusions in inguinal herniorrhaphy specimens may represent müllerian remnants, wolffian remnants, transected vas deferens, or transected epididymis. It is critical to make this distinction because of the potential impact on reproductive function and medicolegal issues.7 Disruption of one vas deferens may generate antisperm antibodies. Classification of glandular inclusions is often subjective, even with experienced pathologists; in one study, interobserver agreement was only 44% to 52% of cases.25 Epididymis typically has a well-formed concentric muscular coat, whereas embryonic remnants lack a muscular coat but have a mantle of fibrous tissue. Some have advocated use of Masson trichrome stain and muscle-specific actin to make this distinction, but this has been refuted by others as inconclusive.25 Comparative analysis reveals that the combination of glandular diameter (with special attention to patient age, recognizing possible changes with advancing development) and histochemical and immunohistochemical stains (trichrome, muscle-specific actin, and CD10) should allow distinction in most cases (Table 14-2). Reliance on light microscopy features alone may be misleading.26 Should inguinal hernia repair specimens be submitted routinely for histopathologic examination? One study of 456 specimens from 371 patients under the age of 20 years revealed 4 unexpected cases with epididymal tissue (1%), leading the authors to conclude that pathologic study was an unnecessary expense.27 In a study of almost 1500 inguinal herniorrhaphies, the authors found vas deferens in 0.13% of cases (see Table 14-2).28 Another report of more than 7000 consecutive pediatric herniorrhaphies found 0.23% vas deferens, 0.3% epididymis, and 0.41% embryonal rests.25 Inguinal hernias characteristically show cremasteric muscle fiber hypertrophy that accounts for the palpable thickening of the spermatic cord (see later discussion of hamartoma).29 This mesothelial-lined cyst results from accumulation of serous fluid between the parietal and visceral tunica vaginalis of the testis (Fig. 14-3). Two variations of a spermatic cord hydrocele occur: the encysted variety, which does not communicate with the peritoneal cavity, and the funicular variety, which does communicate with the peritoneal cavity. The encysted type can be confused with an inguinal mass (lymphadenopathy, hernias), and also primary tumors of the cord.30 A hematocele is the accumulation of blood in the space between the parietal and visceral tunica vaginalis, often in association with hydrocele (see Fig. 14-3). Long-standing hematocele becomes calcified and fibrotic, with numerous hemosiderin-laden macrophages. The causes of hematocele are similar to those for hydrocele. Idiopathic hematoma arising in the spermatic cord or epididymis may be mistaken for neoplasm.31 Varicocele is a mass of dilated tortuous veins of the pampiniform venous plexus of the spermatic cord that occurs posterior and superior to the testis, sometimes extending into the inguinal ring (see Fig. 14-3). The venous plexus normally empties into the internal spermatic vein near the internal inguinal ring; poor drainage and progressive dilatation and elongation result from incompetent valves of the left internal spermatic vein that empties into the renal vein. The right internal spermatic vein is less likely to be involved with varicocele because it drains directly into the inferior vena cava and is less likely to have incompetent valves. Microscopic changes in the pampiniform plexus with varicocele include vascular wall thickening, segmental obliteration, medial hypertrophy of longitudinal smooth muscle fibers, fragmentation of the internal elastic lamina, and occasional occlusive thrombi.7 In contrast to the control group without varicocele, affected patients had significant thickening of the tunica adventitia and tunica media of the spermatic veins (control versus varicocele: tunica adventitia 0.22 ± 0.1 mm versus 0.35 ± 0.08 mm, respectively; tunica media: 0.09 ± 0.04 mm versus 0.25 ± 0.05 mm, respectively).32 Spermatocele is a dilatation of an efferent ductule in the region of the rete testis or caput epididymis.33 The inner lining consists of a single layer of cuboidal to flattened epithelial cells that are often ciliated. The wall is composed of fibromuscular soft tissue, often with chronic inflammation, and the cyst may be unilocular or multilocular (see Fig. 14-3).34 Spermatocele is distinguished from hydrocele by the presence of spermatozoa within the cyst fluid, a distinction that can be made by aspiration cytology. Torsion is a rare complication of spermatocele.7 Mesothelial cyst of the tunica albuginea most often occurs in men older than 40 years, but all ages are affected. It is usually located anterior and lateral to the testis, measuring up to 4 cm in diameter. The cyst is filled with clear or blood-tinged serous fluid, and the lining consists of typical mesothelial cells with a wall composed of hyalinized fibrous tissue. Unilocular and multilocular mesothelial cyst of the spermatic cord is rare and probably arises from embryonic mesothelial remnants such as the processus vaginalis.35 Epidermoid cyst is common in the testis, comprising approximately 1% of testicular tumors, but also may arise, rarely, in the paratesticular area and epididymis.36 Epidermoid cyst consists of a lining of benign keratinizing squamous epithelium and a wall composed of fibrous connective tissue, often with inflammation. Diligent search is required to exclude the presence of adnexal structures or teratomatous elements. Paratesticular epidermoid cyst may arise from squamous metaplasia of wolffian duct structures, displacement of squamous epithelium from the scrotal skin to paratesticular structures during embryogenesis, squamous metaplasia of mesothelial cyst, or monomorphic epidermal development of a teratoma. Epidermoid cyst in the paratesticular area does not recur after surgical excision. Some consider this tumor to be a cholesteatoma when it arises in the epididymis. Dermoid cyst most often involves the testis and paratesticular structures but may occur in the spermatic cord and, very rarely, in the testicular tunics.37 This cyst measures up to 4 cm in diameter and contains soft cheesy yellow-white amorphous material with or without hair and calcifications. The cyst is lined by keratinized squamous epithelium, and the wall contains typical dermal adnexal structures such as pilosebaceous units, although these may be difficult to identify without thorough sectioning. Dermoid cyst does not recur or metastasize after excision. Simple cyst of the rete testis is rare, and is typically unilocular, up to 1 cm in diameter, lined by normal rete testis tubular epithelium, and bulges into the testis proper. When multilocular, it is often associated with epididymal cysts.38 Cystic dysplasia of the rete testis is a benign congenital lesion of newborns and young boys that is frequently associated with ipsilateral renal agenesis and dysplasia.39 It clinically mimics testicular cancer. Long-term follow-up for possible recurrence is recommended. Cystic transformation of the rete testis and epididymis is common in men undergoing dialysis for chronic renal insufficiency. Histologic changes include columnar transformation of the epithelium, accumulation of calcium oxalate crystals, fibrosis, and giant cell reaction.7 Other causes of cystic transformation include mechanical obstruction of the epididymis by tumor or trauma, ischemia, hormonal alterations such as those in hepatic cirrhosis, or cryptorchidism.40 Patients with cryptorchidism display changes in the rete testis referred to in one report as dysgenetic rete testis; changes included metaplastic epithelium with columnar or large cuboidal cells, rete testis hypoplasia, combined hypoplasia and cystic dysplasia, or adenomatous hyperplasia.7 These findings may result from a primary abnormality of the rete testis or incomplete pubertal maturation. A rare case was reported of a 35-year-old with a seminal vesicle cyst that extended through the inguinal canal.41 Epididymitis may be acute or chronic, depending on the inciting agent and the duration of infection.42 It usually occurs in association with orchitis or after trauma but rarely is an isolated finding. Most cases result from retrograde spread by vesicoepididymal urine reflux, but hematogeneous and lymphatic spread account for some cases. Congenital anomalies such as ureteral ectopia may cause epididymitis in infants. The surgical pathologist rarely receives specimens of these diseases. Urethral and epididymal smears and cultures are useful in identifying the causative infectious agent. Patients with acute epididymitis usually present with unilateral painful enlargement of the epididymis, more commonly on the right side, often involving the testicle (50% of cases have epididymo-orchitis) and vas deferens (Fig. 14-4). The epididymis is thickened, congested, and edematous, with white fibrinopurulent exudate in the tubules and stroma. Microabscesses and fistulae may occur, but rupture is uncommon. The tubules may be damaged or destroyed by the inflammation, sometimes with squamous metaplasia and regenerative changes. Acute epididymitis is commonly caused by bacteria. Coliforms account for most cases in children, whereas Neisseria gonorrhoeae and Chlamydia trachomatis are most frequent in young men and Escherichia coli and Pseudomonas predominate in older men.7 Other bacteria that may cause acute epididymitis include Klebsiella, Staphylococcus, Streptococcus pneumoniae, Neisseria meningitidis, Aerobacter aerogenes, and Hemophilus influenzae. The epididymis is a reservoir for N. gonorrhoeae, and although infection may be asymptomatic, microabscesses and edema are common, usually without extensive necrosis. The round cytoplasmic inclusions of C. trachomatis are difficult to identify in routinely stained sections, and immunohistochemical stains, culture, or genotypic studies are usually required for diagnosis. Clinical and histopathologic findings allow separation of some cases of chlamydial and bacterial epididymitis (Table 14-3).43 C. trachomatis–positive cases are clinically indolent, with minimally destructive periductal and intraepithelial inflammation and epithelial regeneration.44 Lymphoepithelial complexes and squamous metaplasia are sometimes present. E. coli–positive cases are characterized by scrotal pain, pyuria, leukocytosis, and highly destructive epididymitis with abscesses and xanthogranulomas. Table 14-3 Comparison of bacterial and chlamydial epididymitis* ESR, Erythrocyte sedimentation rate. *For details see Hori S, Tsutsumi Y. Histologic differentiation and bacterial epididymitis: nondestructive and proliferative versus destructive and abscess forming: immunohistochemical and clinicopathologic findings. Hum Pathol 1995;26:402-407. Viral causes of acute epididymitis include mumps and cytomegalovirus; similar to those causing orchitis. Mumps epididymitis, present in 85% of cases of mumps orchitis, occurs before testicular involvement, usually appearing as unilateral scrotal swelling after parotitis. The epididymis shows vascular congestion, edema, and interstitial lymphocytic inflammation; neutrophils are usually not a prominent feature. Cytomegaloviral epididymitis may occur in patients with AIDS45 or those receiving immunosuppression for transplantation.46 In endemic areas such as India, parasitic infection by Wuchereria bancrofti preferentially involves intrascrotal juxtatesticular lymphatic vessels, with nests of microfilaria with a mean diameter of 0.3 cm2 observable by ultrasonography.7 These infections form epididymal and spermatic cord nodules that contain larvae (microfilariae), eggs, and adult worms, visible in cytologic smears. Early diagnosis and treatment prevent the more severe manifestation of the disease, lymphatic filariasis. Traumatic acute epididymitis is characterized by vascular congestion, petechial hemorrhages, and hematocele. Drugs such as amiodarone also may cause epididymitis.47 Although many cases of acute epididymitis resolve, some become chronic. The epididymis in chronic epididymitis is indurated and scarred, with cystically dilated tubules, marked fibrosis, chronic inflammation, and sperm granulomas; similar changes may account for the late vasectomy syndrome, in which patients report pain many months or years after vasectomy.7 The epithelium shows reactive or metaplastic changes, often with cytoplasmic vacuolization and luminal hyaline aggregates. Epididymitis nodosa, a proliferative lesion of the epididymis, may result from chronic inflammation or trauma, reminiscent of vasitis nodosa.48 Coarse granular cytoplasmic changes appear in the epididymis in the setting of ductal obstruction. Calcification is common in chronic epididymitis, and a foreign body giant cell reaction may occur. Xanthogranulomatous epididymitis also may occur. Special stains for bacteria and fungi may be of value.
Spermatic cord and testicular adnexa
Embryology and normal anatomy
Embryology
Anatomy
Scrotum and testicular tunics
Epididymis
Congenital anomalies
Splenogonadal fusion
Adrenal heterotopia and renal ectopia
Wolffian and müllerian remnants
Appendix testis (hydatid of Morgagni)
Appendix epididymis (vestigial caudal mesonephric collecting tubule)
Paradidymis (organ of Giraldes)
Vasa aberrantia (organ of Haller)
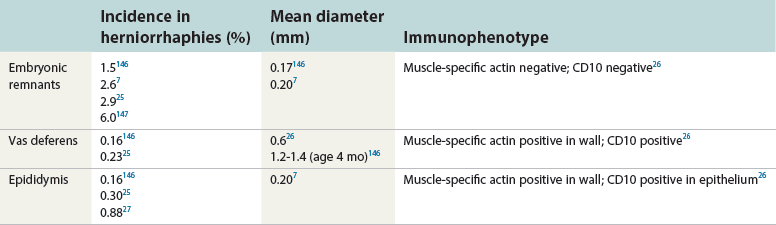
Hernia sac specimens: glandular inclusions versus vas deferens or epididymis
Nonneoplastic diseases of the spermatic cord and testicular adnexa
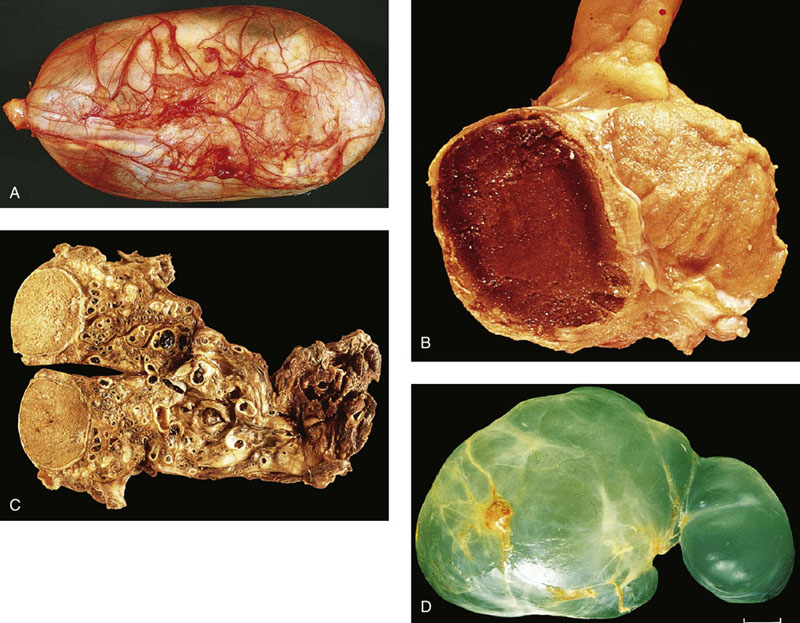
“Celes” and cysts
Hydrocele
Hematocele (hematoma)
Varicocele
Spermatocele (acquired epididymal cyst)
Mesothelial cyst
Epidermoid cyst (epidermal cyst)
Dermoid cyst (mature teratoma)
Simple cyst and cystic dysplasia of the rete testis
Inflammatory and reactive diseases
Epididymitis
Acute epididymitis
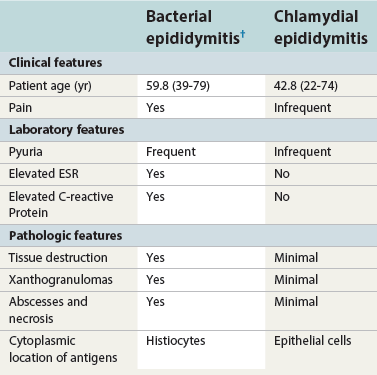
Chronic epididymitis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree