Essentials of Diagnosis
General Considerations
End-stage renal disease (ESRD) represents a major challenge to health care providers around the globe. It is estimated that in 2001 there were approximately 1.1 million people receiving dialysis worldwide and this number is projected to rise by 7% per year to over 2 million by 2010. The financial costs of dialysis provision are considerable and projected worldwide expenditure on dialysis treatment for the decade 2000–2010 is expected to exceed US$ 1 trillion. Whereas chronic dialysis does prolong life in ESRD, it is associated with an annual mortality of approximately 20%, representing a survival rate worse that many common forms of cancer. Renal transplantation does offer improved survival and quality of life, but the majority of patients are not medically suitable for transplantation and a universal shortage of donor organs continues to restrict the number of transplants performed.
Against this background it is critical to appreciate that the majority of cases of ESRD result from the slow progression of chronic kidney disease (CKD) over many months or years. There is therefore an opportunity to intervene in the course of CKD to slow the rate of decline in renal function and thereby reduce the number of patients requiring renal replacement therapy. In this chapter we review the mechanisms that contribute to CKD progression as well as clinical aspects and interventions that are effective in slowing the rate of decline in renal function.
Pathogenesis
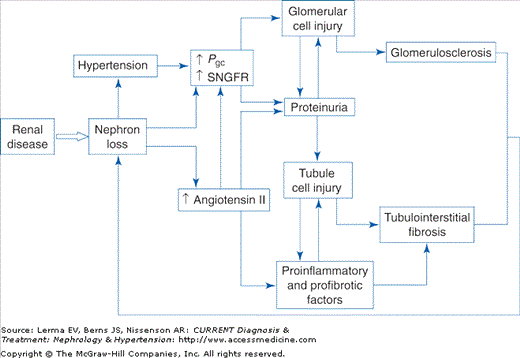
CKD should be viewed as a clinicopathologic syndrome (defined above) that ensues after renal injury resulting from a wide range of kidney pathologies. This suggests that the progressive decline in renal function that is characteristic of CKD results from a common set of mechanisms that is largely independent of the initiating renal pathology. Intensive research over the past three decades has identified several interacting mechanisms that together produce a vicious cycle of progressive nephron loss resulting in ESRD (Figure 22–1).
Studies in animal models of CKD reported that when the nephron number was severely reduced by surgical ablation, marked hemodynamic changes were observed in the remaining glomeruli, characterized by a substantial increase in the filtration rate of each glomerulus (single nephron glomerular filtration rate, SNGFR) that resulted in part from an increase in glomerular capillary hydraulic pressure (Pgc). Whereas these adaptations initially allowed partial compensation for nephron loss, further studies indicated that they were associated with structural injury to glomerular cells and subsequent glomerulosclerosis. In subsequent experiments, treatment with an angiotensin-converting enzyme inhibitor (ACEI) normalized Pgc without abrogating the adaptive increase in SNGFR and prevented glomerulosclerosis, suggesting that increased Pgc (also termed glomerular capillary hypertension) was the hemodynamic factor most likely responsible for glomerular injury. These observations suggested that adaptive hemodynamic changes in glomeruli after substantial nephron loss result in further glomerular damage and nephron loss, thereby establishing a vicious cycle of progressive renal injury.
Angiotensin II is a potent vasoconstrictor peptide that has been identified as an important mediator of the glomerular hemodynamic adaptations observed after nephron loss. Whereas systemic levels of angiotensin II are normal or decreased in CKD, experimental studies have confirmed that intrarenal angiotensin II levels are elevated. Additionally, recent research has identified multiple nonhemodynamic effects of angiotensin II that may contribute to progressive renal injury. These include effects on glomerular permeability resulting in exacerbation of proteinuria, plasminogen activator inhibitor-1 production by endothelial and vascular smooth muscle cells, mesangial cell proliferation and transforming growth factor (TGF)-β expression, macrophage activation, and adrenal production of aldosterone, recently identified as a mediator of renal fibrosis. Taken together, it is clear that angiotensin II plays a central role in the pathogenesis of progressive renal injury through multiple hemodynamic and nonhemodynamic mechanisms. Inhibition of the production or actions of angiotensin II therefore represents a single intervention that may abrogate many of these mechanisms and would be expected to be effective in slowing the progression of CKD.
Proteinuria is usually a result of disordered permselectivity of the glomerular filtration barrier and is therefore the hallmark of glomerulopathy as well as a marker of disease severity. Additionally, research over the past decade has produced evidence that the presence of abnormal amounts of plasma proteins in glomerular ultrafiltrate may contribute directly to further renal damage. In the normal kidney small amounts of low-molecular-weight proteins are present in the tubular fluid and are reabsorbed by proximal tubule cells. In vitro experiments have found that culturing renal tubule cells in the presence of high concentrations of plasma proteins induces expression of a range of proinflammatory cytokines. Moreover, in animal models of renal disease this enhanced expression is evident on the basolateral aspect of tubule cells. It therefore seems likely that these proinflammatory molecules are secreted into the peritubular interstitium where they contribute to the development of interstitial inflammation and fibrosis. Thus, proteinuria provides a mechanistic link between glomerular and tubulointerstitial pathology. Further experimental evidence indicates that abnormally filtered plasma proteins also accumulate within podocytes where they may contribute to glomerular injury.
Prevention
CKD results from a wide range of renal pathologies, many of which occur sporadically; it is therefore difficult to devise an effective strategy for primary prevention of CKD. One important exception to this principle, however, is diabetic nephropathy, the most common cause of CKD in many developed countries. In this case the “at-risk” population may be readily identified and at least two interventions have been shown to reduce the incidence of diabetic nephropathy. Following the landmark Diabetes Control and Complications Trial (DCCT) it became clear that the level of glycemic control is critical in determining the risk of microvascular complications, including nephropathy. Among patients in the intensive diabetic control group, significantly lower hemoglobin A1C (HbA1C) levels versus conventional therapy (7.2% versus 9.1%) were associated with a 34% reduction in the risk of developing microalbuminuria. Second, treatment with an ACEI may reduce the risk of developing microalbuminuria. One small study found some beneficial effect (absolute risk reduction of 12.5%) of ACEI treatment among normotensive, normoalbuminuric patients with type 2 diabetes and recently a large randomized trial (BENEDICT) that included 1204 patients with type 2 diabetes and hypertension reported a significantly lower incidence of microalbuminuria among patients treated with trandolapril (6.0%) or trandolapril plus verapamil (5.7%) versus those treated with verapamil (11.9%) or placebo (10%). Thus tight glycemic control (HbA1C <7%) and treatment of hypertension with an ACEI should be regarded as essential interventions for preventing nephropathy in patients with diabetes.
For other forms of CKD the most critical factor for improving outcomes is early detection to allow intervention with measures that slow the rate of decline in renal function. Appropriate tests for CKD screening will vary according to circumstances and available resources. As most forms of CKD are associated with urinary abnormalities, the simplest form of detection is urinalysis with standard urinary dipsticks. Some forms of CKD may not cause urinary abnormalities and screening should therefore also include an estimate of renal function based on a measurement of serum creatinine. Diagnostic tests and further investigations are discussed in more detail below.
As CKD is relatively uncommon in the general population, it would be expensive and inefficient to screen entire populations. On the other hand, epidemiologic studies indicate that substantial numbers of patients have undiagnosed CKD. Focused screening of “at-risk” patients would therefore increase the detection of early stage CKD and facilitate early intervention to preserve renal function. Factors that place patients at risk for CKD vary in different countries, but general categories of patients who should be considered for screening are listed in Table 22–1. The optimal interval for screening remains to be determined but annual screening in high-risk groups is recommended.
|
Clinical Findings
CKD is often asymptomatic until moderate to severe renal failure has developed. Nocturia due to a failure of urine-concentrating mechanisms may be an early symptom of CKD, but this is often attributed to prostatism and rarely prompts patients to seek medical attention. If severe proteinuria is present, peripheral edema may be the first symptom to develop. Patients with advanced renal failure may present with malaise, breathlessness, pruritis, loss of appetite, nausea, and vomiting.
Similarly, abnormal clinical signs are often lacking in patients with CKD. Hypertension is almost universally present and is often the first detectable sign. It is thus critical that all patients with newly diagnosed hypertension be screened for CKD. Peripheral edema may be present in patients with severe proteinuria or more advanced renal failure. Clinical pallor due to anemia may be observed in patients with CKD stage 3–5. Most (but not all) patients with CKD have urinary abnormalities detectable with standard dipstick testing. Proteinuria is the hallmark of CKD and may be accompanied by hematuria.
Laboratory investigations are essential for the diagnosis of CKD. As discussed previously, the diagnosis rests on the detection of urinary abnormalities (usually proteinuria) and impaired renal function.
The measurement of protein or albumin concentration in a 24-hour urine collection represents the most accurate method for assessment of proteinuria, but clinical utility is limited by inconvenience to patients and variable success in achieving a complete collection. Whereas protein or albumin concentration in a random urine specimen is of limited use due to variations in urine osmolality, the urine protein or albumin-to-creatinine ratio provides a reliable measure of proteinuria. Moreover, the urinary protein-to-creatinine ratio expressed in milligrams/milligram correlates closely with 24-hour urinary protein excretion expressed in grams/day and is thus convenient for patients as well as being easy to interpret. Some authorities prefer to use the albumin-to-creatinine ratio. Due to diurnal variations in the urinary protein excretion rate the assessment should be performed on an early morning specimen of urine.
Determining the optimal method for the assessment of GFR depends upon finding a method with the best compromise between accuracy and reproducibility as well as patient convenience. Radioisotope clearance studies represent the most accurate method, but these require a hospital attendance of several hours as well as some radiation exposure and are therefore not suitable for serial monitoring of renal function. Creatinine clearance studies based on 24-hour urine collections were widely used until recently, but are inconvenient for patients and results may be significantly affected by undercollections or overcollections of urine. These difficulties prompted the development of a variety of formulas for estimating GFR based on serum creatinine concentration. Comparison with radioisotope clearance studies has shown that a four-variable equation [GFR = 1.86.3 × (Pcr in mg/dL)−1.154 × (age)−0.203
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree