and Hubert Lepidi1
(1)
UER Médecine, Aix-Marseille Université, Marseille, France
Abstract
The bulbo-clitoral organ is, such as we have seen previously, provided with a significant innervation. The sensory somatic fibres form a very important contingent in this innervation. They also make it possible to permanently inform the overlying nerve centres of any stimulus occurring at the level of this organ. Very specialised and specific receptors (“corpuscles”), positioned at the ends of some of these sensory fibres, receive the stimuli corresponding to “sensuous sensations” and transmit them to the nerve fibres, which convey them to the sensitive and specialised cerebral areas. Nerve endings are extremely numerous at the level of the bulbo-clitoral organ, especially the glans, which explains the extreme sensitivity of the latter. It should be noted from the start that although the clitoral glans has the same number of sensory “terminations” and genital corpuscles as the penile glans, assessed at around 8,000, the density of these receptors, with respect to the size of each of these organs, is 50 times higher for the female glans. This means that the sensitivity of the clitoral glans is extreme compared to that of the male glans, which is already very high!
9.1 General
The bulbo-clitoral organ is, such as we have seen previously, provided with a significant innervation. The sensory somatic fibres form a very important contingent in this innervation. They also make it possible to permanently inform the overlying nerve centres of any stimulus occurring at the level of this organ. Very specialised and specific receptors (“corpuscles”), positioned at the ends of some of these sensory fibres, receive the stimuli corresponding to “sensuous sensations” and transmit them to the nerve fibres, which convey them to the sensitive and specialised cerebral areas. Nerve endings are extremely numerous at the level of the bulbo-clitoral organ, especially the glans, which explains the extreme sensitivity of the latter. It should be noted from the start that although the clitoral glans has the same number of sensory “terminations” and genital corpuscles as the penile glans, assessed at around 8,000, the density of these receptors, with respect to the size of each of these organs, is 50 times higher for the female glans. This means that the sensitivity of the clitoral glans is extreme1 compared to that of the male glans, which is already very high!
These terminations and corpuscles make the clitoris, and especially the glans, an extraordinary organ, which is very specialised, and exclusively dedicated to female pleasure, whereas the penis and the penile glans are “multifunctional” as they are also used for urinating purposes, penetration and spermatic emission during the coitus act.
It should be noted that clitoral sensory corpuscles have been observed on animals (studies conducted by J.F. Tello on female mice, rats and ewes and by D. Ohmori on female rabbits, bitches and cats). However, these authors acknowledge that the genital corpuscles of the females under study are infinitely simpler than those of the human clitoris!
Several types of nerve endings (sensory nerve “terminations”) can be identified in the human clitoris:
Some are not specific to the clitoris and are similar to tactile terminations, the same nerve endings as those identified over all of our skin and more particularly in areas dedicated exteroception: palms of the hands, soles of the feet and labia majora.
Among these terminations, some do not have any corpuscles: These are free terminations, “free nerve endings” and there exist others, which end at a receptor corpuscle or a tactile corpuscle; these terminations are referred to as encapsulated terminations, “corpuscular nerve endings”. In this case, it is the unit consisting of the nerve fibre termination and the corpuscle, in which this termination has developed, which forms the receptor. Such as over the entire skin surface, the corpuscles observed at the clitoris are standard tactile corpuscles: Meissner’s corpuscles, Ruffini’s corpuscles and Pacini’s corpuscles.
Other hyper-specialised corpuscles, specific to the clitoris, Krause-Finger’s corpuscles or pleasure corpuscles.
All these nerve endings have a certain number of similar characteristics:
They are part of the general context referred to by J.F. Tello as “sensory terminations of external genitals”, which are the “mucocutaneous specialised sensory end organs” of the English authors (R.K. Winkelmann).
They only appear at late stage and develop at puberty (Winkelmann), which suggests a hormonal influence. The free nerve endings and Pacini’s corpuscles are an exception to this rule as they can be observed and are already perfectly formed (J.F. Tello) in newborn children (R.K. Winkelmann) and even in foetuses (K.E. Krantz).
Their degree of organisation and their complexity tend to increase with age (R.K. Winkelmann) and the following issue can arise (such as mentioned by H. Jaeger) “certain repercussions of the intensity of sexual life on the development of these formations”.
They are more or less numerous according to the part of the external genitalia being considered, the clitoris being incontestably the organ which has the greatest number.
Their number and their distribution vary according to individuals.
We will successively study non-specific nerve endings and specific nerve endings.
9.1.1 Non-specific Nerve Endings
Among the non-specific nerve endings, terminations in direct connection with the skin’s epidermis and terminations located in the dermis can be mentioned.
Nerve endings connected to the epidermis: These include, on the one hand, free nerve endings and, on the other hand, terminations with Merkel discs.
The free nerve endings correspond to axons, which exit their myelin sheath (Schwann’s cell envelope), ramify and end in the dermis, in contact with the epidermis (Fig. 9.1), or in the epidermis itself, if they have crossed the lamina basalis, which remains exceptional (H. Jaeger). Small terminal swelling or bulbs may be present at the end of the ramifications (K.E. Krantz). They can anastomose to form, at the most superficial part of the dermis, a subepidermic nerve reticulum (H. Jaeger). They are distributed as thermoreceptors (sensitive to thermal variations, especially if they are sudden) and nociceptors (sensitive to pain). The extreme density of the distal axons and their arborisation and ramifications, which can be observed on histological preparations, account for the abundance of these free nerve endings, not only at the level of the clitoris but also of the labia minora.
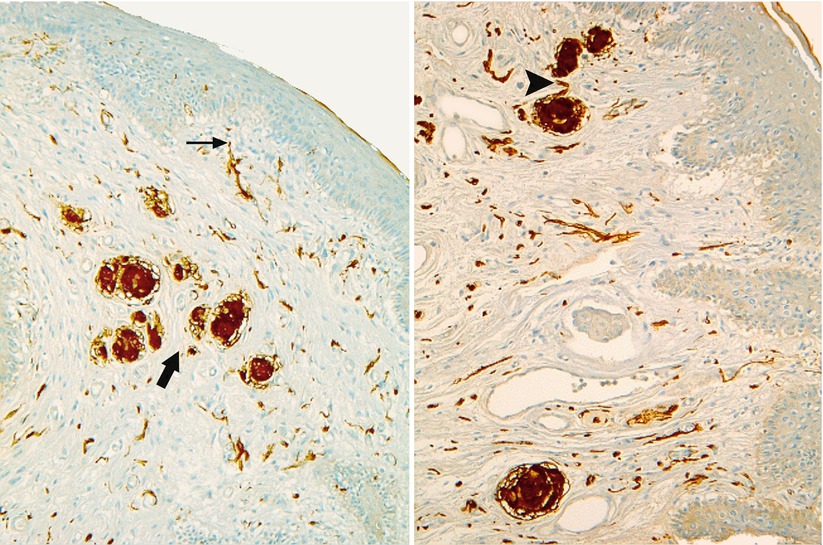

Fig. 9.1
Free nerve endings and bulbous corpuscles (PS100 staining). Note: A free nerve ending at the top of a dermal papilla (thin black arrow). A group of corpuscles (thick black arrow). The axon penetrating into the corpuscle (black arrowhead)
The meniscoid corpuscles (discs of Merkel or “tactile menisci”) are very superficial, in contact with the basal epithelium of the glabrous skin’s epidermic ridges. They consist of axon ramifications, whose dilated ends come in contact with a particular meniscoid cell: the Merkel cell. C.J. Cold and J.R. Taylor observed them on the glabrous part of the external epithelium of the prepuce, i.e. at the level of the clitoral hood. Some are also present at the level of the labia minora. These discs detect localised light pressures and remain sensitive throughout the duration of this pressure. They therefore are rapidly adapting mechanoreceptors with an extended action.
Dermic nerve endings: They are Meissner’s corpuscles and Ruffini’s corpuscles.
The encapsulated tactile corpuscles (Meissner’s corpuscles) are also rapidly adapting mechanoreceptors. They have an alveolar aspect and are located in the top part of the dermis of the glabrous skin, in the middle of the dermis papillae. As they are sensitive to the slightest touch (they are capable of appreciating the speed at which a pressure is applied!) and therefore to brushing, they are also capable of detecting the slightest inequalities. They consist of spiral nerve ramifications, within a stack of lamellar cells, the lemnocytes, which are Schwann’s cells and which are flattened and superimposed like piles of plates. They have a fibrous capsule (collagen fibres). They are especially observed on the medial surface of the labia minora and, in a smaller number, at the level of the clitoral hood.
Ruffini’s corpuscles are located at a deeper level, in the dermis of the prepuce and of the labia minora. They are at both mechanoreceptors and proprioceptors. They are ovoid formations consisting of collagen fibres, between which axon ramifications having penetrated the corpuscle have developed. A nerve fibre exiting the corpuscle to reach the epithelium, as a free termination, or to end in a nearby Meissner’s corpuscle (K.E. Krantz) is sometimes observed at their level. They are positioned parallel to the skin. They are slow adapting corpuscles, sensitive to cutaneous stretching (e.g. during shaving) or to an extended pressure (as they appreciate the related intensity and duration).
Dermo–hypodermic nerve endings (present in the deep dermis and the hypodermis): These are the corpuscles discovered by Vater and then studied by Pacini,2 a few years later (Vater-Pacini’s corpuscle), which are now referred to as lamellar corpuscles. Their presence in significant numbers at the level of the clitoris deserves an in-depth description. These corpuscles are often large (transverse diameter of 5–6.5 μm) and are both mechanoreceptors and proprioceptors. They have an ellipsoidal shape, and their section is generally oval or circular even if we have observed irregular aspects (Fig. 9.2), polygonal on certain sections. They can be isolated and positioned at a certain distance from each other. They are often routed in groups of two, in similar sizes, or on the contrary, in very different sizes. They can also be grouped in clusters, in a half circle or even in a single line (Fig. 9.3). On a structural level (Fig. 9.4) the corpuscle consists of several cellular rings “the lamellae”, concentric as an onion bulb and surrounding a central axon, which has lost its myelin sheath by penetrating into the axial space. The peripheral conjunctive envelope, as well as the external lamellae (capsule), is within the extension of the perineurium of the axon. The internal lamellae (teloglial lamellae), forming the intermediate growth area, consist of flattened cells, resembling fibroblasts but originating from Schwann’s cells, which are more closely applied than the external laminae. Even more internal and more closely arranged lamellae form the “central club” of the corpuscle (“core” according to the Anglo-Saxon authors), in which is located the receptor nerve fibre, ending with a bulge. In electronic microscopy, these internal lamellae are only half-lamellae facing each other and separated by a radial slit. The total number of lamellae in a corpuscle is variable (20–30 on average in an adult). However, around 30 or more is frequent (Figs. 9.2 and 9.4). There are 5–10 lamellae in a foetus; this number increases with time (growth in the area of the teloglial lamellae) and, in particular, during puberty. As of birth, the lamellar corpuscles are already formed (J.F. Tello): It is already possible to identify the external lamella/strips separated by wide spaces and the internal lamellae, which are very close to each other, especially when approaching the centre. For this author, at puberty, the corpuscles will increase in volume, the number of lamellae will increase, the lamellae will tend to become closer to each other and the central area will generally tend “to be reduced to its simplest expression”. However, corpuscles with a larger core can be observed in adults. It should be recalled that the inter-lamellar spaces of a mature corpuscle are not empty but filled with a viscous liquid film, in which collagen fibrils can be observed by electronic microscopy (C. Cavallotti et al.). The central axon, which has penetrated the corpuscle, is routed up to the core end and often ends in by one or more bulges. Along its route in this core, irregularities, varicosities and thickness inequalities are also observed on this axon (H. Jaeger). For J.F. Tello, the presence of corpuscles as of birth and the fact that they are formed in foetuses as of the 6th month, in contact with the nerve components, should be explained by their role in the early acquisition of protopathic sensitivity.
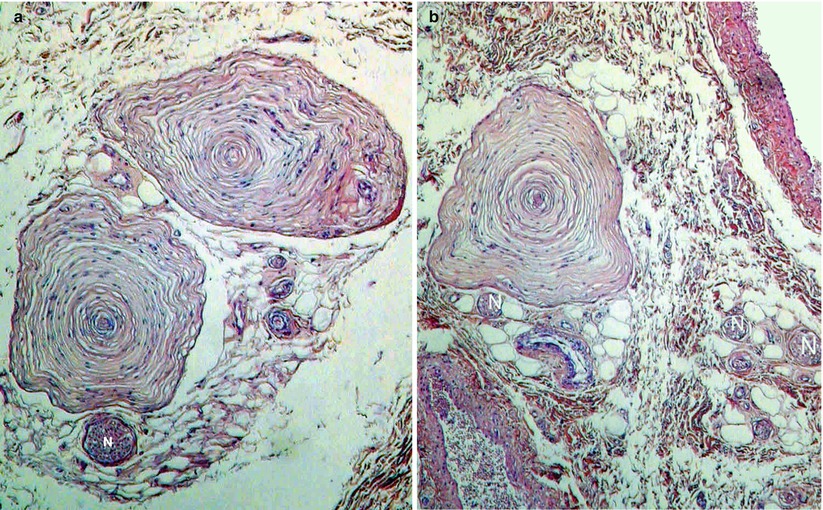
Fig. 9.2
Pacinian corpuscles in the hypodermis of the female prepuce (HPS staining). Observe: The nerve trunk (N) adjoining one of the two lamellated corpuscles (left picture). The numerous concentric in shape successive lamellae of each corpuscle. The central naked axon in each core. The adipose tissue in which you can find the lamellated corpuscles
In the clitoris, lamellar corpuscles can be found in various locations: Firstly, in the vicinity of nerve cords (nerve ramus developed from the branches of the pudendal nerve), which they follow more or less closely, sometimes even inside the epineurium of the nerve (Fig. 9.5), especially to transmit the slightest vibratory excitation. They may even be positioned alongside the nerve endings to form a sort of sensitive pack. It is in the retro-crural small space (in the cell tissue limited by the retro-crural fascia) and in the latero-cavernous areas of the descending part of the body (Fig. 9.3) that their presence is the most obvious. They are also found in the dermis (especially the deep dermis) but the favoured sites are the subcutaneous cell tissue and the hypodermis, in contact with fatty tissue (especially at the level of the prepuce), and the suspensory ligament of the clitoris (see Chap. 12). A small number also exists in the albuginea of the cavernous bodies and more exceptionally in the septula of the cavernous bodies (K. Yamada). Such as we have observed, it is primarily the clitoral part of the bulbo-clitoral organ, which is equipped with lamellar corpuscles. However, it is not the only part to be provided as such. Lamellar corpuscles are identified at the end of the residual spongy part and glans, which form the distal part thereof (E. Lastly); the presence of lamellar corpuscles in the epithelium of the prepuce and at the labia minora (K.E. Krantz) is also to be recalled. It should be noted that all the above-mentioned formations have a greater amount of lamellar corpuscles than the penile glans or the male prepuce (C.J. Cold and J.R. Taylor).
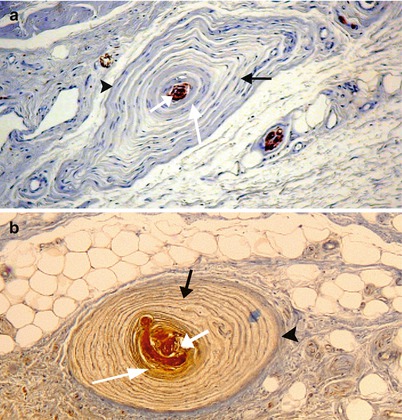

Fig. 9.3




Usual places of the clitoral lamellated corpuscles (The blue arrows show the corpuscles). (a) A corpuscle inside the epineurium (connective sheath of the nerve). The corpuscle occupies the place of a bundle of axons and is surrounded, just like it, by a sort of perineurium confounded with its peripheral connective layer. (b) Three lamellated corpuscles inside the cellular tissue around the tunica albuginea (Alb) of the corpora cavernosa (CC). (c) Lamellated corpuscles at the level of the junction of hypodermis with dermis. (d) A lamellated corpuscle inside the adipose tissue of the preputial hypodermis. (e) Several corpuscles in single file, inside the basis of the glans clitoridis. Note the different shapes of the corpuscles: Classical, oval-shaped (a), rounded (b, e), triangular (c), bludgeon-shaped or “mussel-shaped” (d), (evocative forms of seafood are frequent)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








