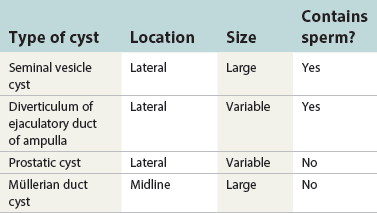
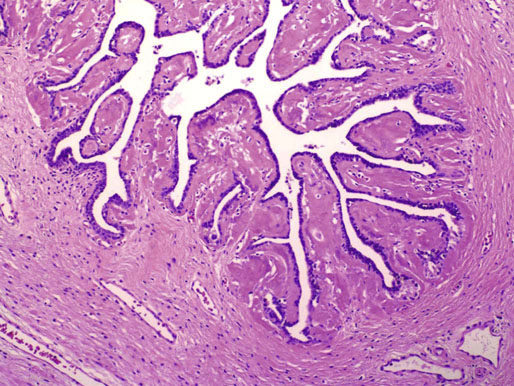
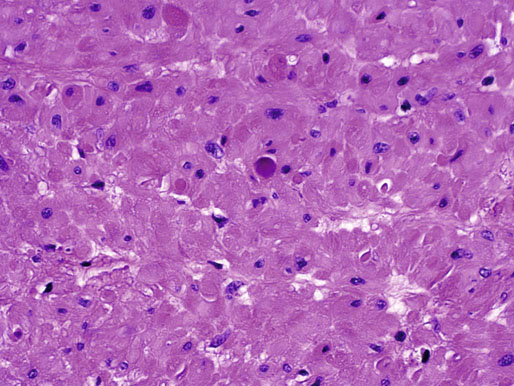
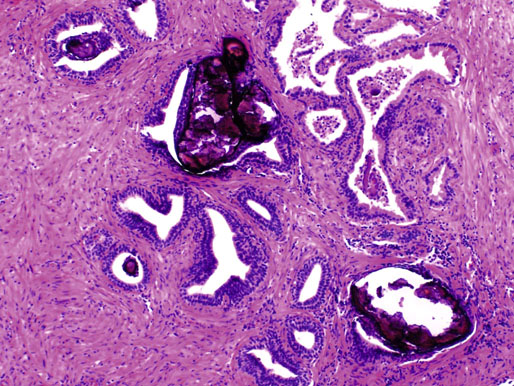
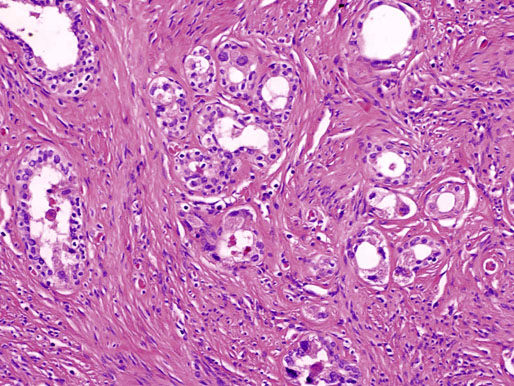
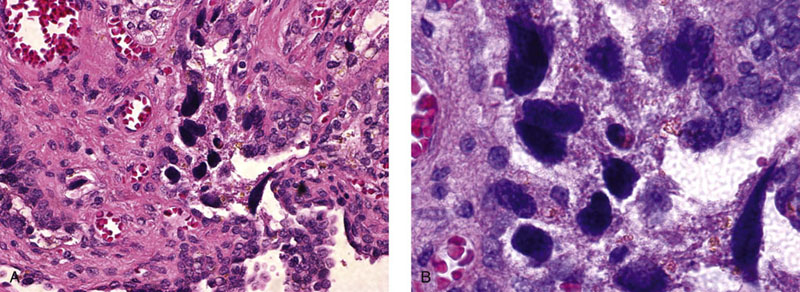
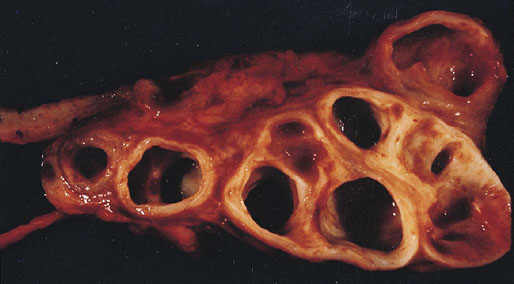
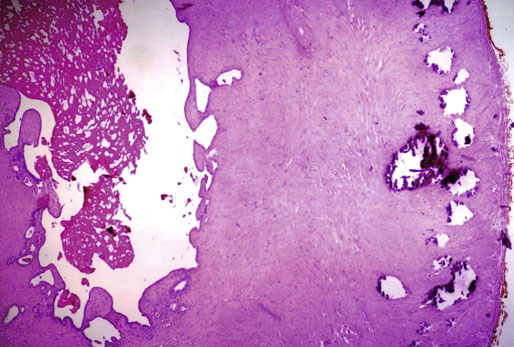
Chapter 10 The seminal vesicles were described by the Italian anatomist Berengario a Carpi in 1521. These paired androgen-dependent accessory sex glands were first regarded simply as storage sites for semen, but their milky alkaline secretions are now known to constitute the majority of the ejaculate, thus promoting sperm function and providing a variety of potent antibacterial factors to the male genital tract.1–3 Infections, cysts, and neoplasms of the seminal vesicles are rare, in sharp contrast to their anatomic neighbor, the prostate. Cross-sectional imaging modalities, including ultrasonography, computed tomography, and magnetic resonance imaging (MRI), have been increasingly used for evaluation of the seminal vesicles and vasa deferentia.4 Under the influence of testosterone, the seminal vesicles appear during the thirteenth week of development as outpouchings of the lower mesonephric ducts.5 They are bounded by the prostate distally, the base of the bladder anteriorly, and Denonvilliers fascia and the rectum posteriorly. Their anatomic position in this region is variable, and they are sometimes found within or adherent to the posterior capsule of the prostate gland.2,3,6 The seminal vesicles may be palpable on digital rectal examination, and, when adherent to the prostate, may be mistaken for prostatic nodularity or induration. Approximately 5% of specimens from prostate biopsies for nodularity contain fragments of seminal vesicle epithelium, a potential source of diagnostic confusion (Fig. 10-1).7,8 In adults, the seminal vesicles average 6 cm long and 2 cm wide, with a capacity of up to 4.5 mL, although size, shape, and volume vary widely.6 Fig. 10-1 Tangential needle biopsy through the seminal vesicles, which may be mistaken for adenocarcinoma. The muscular wall of the seminal vesicles consists of a thick, circumferential coat of smooth muscle, which contracts during ejaculation. Contraction is regulated by excitatory adrenergic and modulatory neuropeptide Y–encephalin-peptidergic nerve fibers.6 Tangential cuts through this wall frequently reveal irregular clusters of epithelial tubules, which may be mistaken for adenocarcinoma. The ducts of the seminal vesicles merge with the ampullae of the vasa deferentia on each side to form the ejaculatory ducts, and these structures compose a functional unit that develops slowly until the onset of puberty.6 These ducts immediately enter the central zone of the prostate and converge as they approach their outlets at either side of the verumontanum in the prostatic sinus of the prostatic urethra. Unlike the seminal vesicles, the ejaculatory ducts lack a thick muscular wall, surrounded by a collagenous stroma. Luminal and wall dimensions are remarkably uniform among adult men, with diameter greater than 2.3 mm the cutpoint for dilatation.9 Histologically, the seminal vesicular mucosa consists of complex papillary folds and irregular convoluted lumina lined with nonciliated, pseudostratified tall columnar epithelium. The cells are predominantly secretory, containing microvesicular lipid droplets and characteristic lipofuscin pigment granules.10 The pigment is golden brown and refractile, increasing in amount with age; similar pigment may be seen in prostatic epithelium, but it is usually less conspicuous and abundant.11 These cells also contain androgen receptors, similar to the prostatic epithelium. Secretory products include glycoproteins, protein kinase inhibitor, protein C inhibitor, fructose, prostaglandins, ascorbic acid, sperm motility factor, transferrin, lactoferrin, lysozyme, and metallothionein. Secretion is regulated by nerves from the pelvic plexus, which are cholinergic postganglionic, sympathetic, and possibly parasympathetic.6,12,13 Up to 85% of the seminal fluid originates in the seminal vesicles, and the semen volume varies from 2 to 5 mL. It takes 3 days for the epithelium to refill the seminal vesicles after ejaculation. The immunohistochemical profile of the normal seminal vesicle epithelium includes the following (maximum staining 3+): pancytokeratin (AE1/AE3, CAM 5.2) +++, cytokeratin (CK) 5/6 +++, CK34βE12 −, CK7 +++, CK8 +, CK14 −, CK18 +++, CK19 +++, CK20 −, Ki67 = 1%, p53 −, p63 +++, neuron-specific enolase (NSE) −, carcinoembryonic antigen (CEA) −, epithelial membrane antigen (EMA) −, CA 19-9 −, estrogen receptors −, progesterone receptors −, HER2 +, HepPar1 −, CD34 −, CD10 +, prostate-specific antigen (PSA) −, prostate acid phosphatase (PAP) −, prostate-specific membrane antigen (PSMA) −, racemase −, desmin −, ASMA −, CD68 −, S100 −, CD45 −, synaptophysin −, thyroid transcription factor-1 (TTF-1) −, CDX2 −, mucin 1 (MUC1) −, MUC2 −, MUC5AC −, MUC6 +++, CD56 −, PAS −, dPAS −, and Alcian blue +.14 MUC6 and PAX2 are selectively expressed in benign seminal vesicle epithelium, in contrast to benign prostate and adenocarcinoma.15,16 The seminal vesicles begin to shrink in the seventh decade.17 The tall columnar cells lining the mucosa in young men are replaced over time by flattened cuboidal cells, comprising only 50% of the epithelium in men in the fifth decade of life and 2% in octogenarians. With advancing age, the stroma of the seminal vesicles becomes hyalinized and fibrotic and may exhibit lipofuscinosis, similar to the epithelium.18 The flattening of the epithelium is accompanied by striking nuclear abnormalities, and highly atypical cells are present in approximately 75% of older men (Fig. 10-2).19–26 These cells have large, irregular hyperchromatic nuclei with coarse chromatin and prominent nucleoli. Multinucleated cells are also present, as well as giant ring-shaped nuclei with large intranuclear cytoplasmic inclusions. Mitotic figures are absent. The immunophenotype of the atypical cells is similar to that of the benign seminal vesicle epithelium, but it tends to be weaker in intensity.14 Fig. 10-2 Seminal vesicle from an 80-year-old man that shows distinctive highly atypical hyperchromatic epithelial cells. A, Low power. B, Oil immersion power. These nuclear abnormalities, not observed before 20 years of age, are probably degenerative changes reflecting hormonal influences. When encountered in needle biopsy specimens, such “pseudomalignant” cytologic atypia may lead to a mistaken diagnosis of prostate cancer.27 Difficulty may also be encountered in cytologic evaluation of fluids obtained by prostatic massage because seminal vesicular cells are frequently shed intact into the lumina.28 The distinctive lipochrome pigment aids in their recognition.21–23,25 Cells in prostatic aspirates derived from the seminal vesicles and ejaculatory ducts may be cytologically indistinguishable. DNA ploidy analysis reveals aneuploidy in up to 48% of seminal vesicles.19,29 Consequently, DNA analysis of prostate cancer specimens may yield false-positive results if contaminated by seminal vesicle tissue. It is uncertain why there is such a low level of aneuploidy in an organ with frequent and substantial cytologic atypia. Seminal vesicular cells are found as contaminants of cervical smears in 10% of specimens with spermatozoa, and they may be diagnostically confusing.30 These cells contain foamy cytoplasm, scant pigment, vesicular hyperchromatic nuclei, sievelike chromatin pattern, and mild anisokaryosis. Malformations of the seminal vesicles are frequently associated with abnormal development of other mesonephric derivatives, although isolated hypoplasia, agenesis, and cysts have been reported.31,32 Unilateral absence of one seminal vesicle may be associated with ipsilateral prostatic central zone agenesis,33 renal agenesis, or vas deferens anomalies or agenesis.34 Unilateral agenesis is often associated with decreased semen volume, hypospermia or azoospermia, impaired sperm motility, acidic ejaculate, and absence of fructose and coagulation activity. Up to 37.5% of these men are infertile, a finding implying that the single vas is abnormal. Bilateral dilation or absence of the seminal vesicles is sometimes observed in patients with cystic fibrosis, reportedly caused by an unexplained failure of development.35 Unilateral duplication of the seminal vesicles is an unusual anomaly. Seminal vesicle surgery is usually undertaken for evaluation of congenital malformations. Maldevelopment of the ureteric bud results in ureteral ectopy, with the ureters terminating in the seminal vesicles, prostatic urethra, vas deferens, epididymis, or ejaculatory ducts.36–50 Ureteral ectopy is frequently seen in association with ipsilateral renal dysgenesis or contralateral renal hypertrophy, and the seminal vesicles become enlarged and dilated with accompanying ureterocele. Seminal vesicle cysts are rare and may be congenital or acquired.51–54 Symptoms are vague, including perineal pain during ejaculation or defecation, dysuria, urinary retention, and recurrent epididymitis. Congenital cysts are associated with ipsilateral renal agenesis in 80% of cases and commonly with ureteral ectopia or agenesis (Zinner syndrome); more than 100 cases have been reported.36,53,55–66 These paired anomalies are caused by the close association of the ureteric bud and mesonephric duct during embryogenesis; the ureteric bud is more cephalad, and the elongate ureter may fail to connect with and stimulate the differentiation of the nephrogenic blastema. Other cases of congenital cysts are associated with ipsilateral dysplastic kidney,67 ipsilateral absence of the testis,40 or hemivertebra.68 Autosomal dominant polycystic kidney disease carries a high risk (43%) of coexistent seminal vesicle cyst.69 Congenital cyst of the seminal vesicle is usually detected in patients between 18 and 41 years of age, the period of maximal sexual and reproductive activity; most of these cysts arise in white patients.70 Cyst may be asymptomatic or cause ill-defined lower urinary tract symptoms.71 MRI is useful for detecting cyst and associated urogenital tract anomalies.72 The cyst is usually unilateral and unilocular, lateral to the midline, up to three times larger than the normal seminal vesicle, and considerably smaller than müllerian duct cyst (Table 10-1), although rarely the cyst is gigantic73–75 and may cause rectal obstruction.76 Enlargement is caused by insufficient drainage with accumulation of seminal fluid. The unilocular cyst contains viscous pale-white fluid, similar to the usual secretions of the seminal vesicles, and is lined by cuboidal or flattened epithelium with a fibrous wall of variable thickness. Rarely, intracystic papillary adenoma may be present.77 Massive enlargement has been called hydrocele or hydrops.78 Bilateral congenital cysts are rare and may be associated with absent vasa deferentia.79 One case report described a 28-year-old patient with cystic dilatation of the terminal seminal vesicles associated with dilated ejaculatory ducts and utricle.80 Acquired cyst is usually associated with inflammation and obstruction of the ejaculatory ducts and seminal vesicles (Fig. 10-3). This fluctuant cyst may be palpable on digital rectal examination and often contains red blood cells, white blood cells, and spermatozoa. The epithelial lining is inflamed or sloughed, depending on the duration and severity of inflammation. In one case, endoscopic removal of a small calculus lodged at the orifice of the ipsilateral ejaculatory duct caused complete resolution of a 14-cm seminal vesicle cyst, evidence for an obstructive etiology and demonstration of the utility of preoperative imaging techniques to detect stones.81 One unique case manifested as an inguinal hernia, with the cyst extending through the inguinal canal.82 A fatal case of Klebsiella seminal vesiculitis in a middle-aged man was associated with concurrent pneumonia.83 Fig. 10-3 Incidental acquired cystic dilatation of the seminal vesicles found at autopsy in a 70-year-old man. Echinococcal (hydatid) cyst can occur in the retrovesicular region, invariably in association with infection in another organ; cyst excision is curative.84–89 Megavesicles are characterized by marked dilation of the seminal vesicles. The cause of megavesicles is unknown, but this condition is sometimes seen in diabetic patients.90 Cystadenoma is a benign neoplasm mimicking acquired cyst. The differential diagnosis of seminal vesicle cyst includes prostatic cyst,91 ejaculatory duct diverticulum, cystic dilation of wolffian and müllerian duct remnants, and cystic lymphatic malformation originating from the mesentery of the sigmoid colon (see Table 10-1).90,92–94 The cyst may produce hydronephrosis caused by displacement of the lower ureter toward the midline with obstruction. Radiographic evaluation of seminal vesicle cyst includes vasoseminovesiculography, ultrasonography, computed tomography, and MRI.61,95 Aspiration of congenital or acquired cyst is diagnostic and relieves symptoms,96 but surgical removal or marsupialization is preferred. Laparoscopic excision may be associated with lower morbidity.97 Benign ectopic prostatic tissue rarely arises in seminal vesicle tissue and may be mistaken for extraprostatic adenocarcinoma.98 Ectopia may involve prostatic or urothelial tissue and most frequently is identified in the wall of seminal vesicle cyst, sometimes forming a nodular mass.99,100 The intimate spatial coexistence of endoderm-derived prostatic tissue and mesonephric duct–derived seminal vesicle tissue is rare.101 Localized amyloidosis of the seminal vesicles (senile seminal vesicle amyloidosis; Fig. 10-4) is observed at autopsy in 5% to 8% of men between 46 and 60 years of age, in 13% to 23% of men between 61 and 75 years old, and in 21% to 34% of men who are older than 75 years.102–104 The clinical incidence in men with hemospermia is 33%105; at radical prostatectomy, the incidence is 1.1% to 4.7%.106,107 There may be an association with prior androgen deprivation therapy for prostate cancer,108 but this has been refuted.106 The mean thickness of the amyloid band did not correlate with the patients’ age, preoperative prostate-specific antigen levels, prostate weight, or the Gleason score and stage of prostate cancer, and amyloidosis did not provide an absolute or relative protection from seminal vesicle invasion.109 Amyloidosis usually extends bilaterally along the ejaculatory ducts and vasa deferentia to form linear or massive nodular subepithelial deposits of amorphous eosinophilic fibrillar material. Basement membrane thickening is observed, and deposits may be seen within the vesicular lumina, occasionally causing significant luminal narrowing. Rare cases are associated with calcification or a florid foreign body giant cell reaction.110 By contrast, systemic amyloidosis infrequently affects the seminal vesicles, involving vascular walls, smooth muscle, and stroma.111 Vesicular amyloidosis is usually asymptomatic, but it may cause hematospermia or chronic perineal pain, or it may mimic seminal vesiculitis.112,113 It is best visualized by MRI and may mimic tumor invasion from bladder or prostate cancer.105,114–116 Localized and systemic amyloidosis may coexist.117 Special stains that confirm the diagnosis of amyloid include the following: Congo red, which appears red by light microscopy with apple-green polarization birefringence; methylene blue, which reveals green polarization birefringence; crystal violet and toluidine blue, which impart a metachromatic appearance to the deposits; and periodic acid–Schiff (PAS) and Alcian blue stains, which are weakly to moderately positive. The composition of localized seminal vesicle amyloid is histochemically unique (permanganate sensitive, lactoferrin and amyloid P component positive, and non-AA, non–β2-microglobulin [non-β2M], non-κ or λ, nonprealbumin type105), apparently derived from secretory protein of the seminal vesicles, identical in sequence to the N-terminal portion of semenogelin.118 Amyloid at other sites is derived from light chains or serum amyloid protein.104,110,117,119,120 Small, 15- to 20-µm eosinophilic hyaline bodies are sometimes observed within the muscular wall of the seminal vesicles, vas deferens, and prostate and are designated stromal hyaline bodies (Fig. 10-5).24,121,122 These round to oval structures probably result from degeneration of smooth muscle fibers, and transition forms can be seen arising from smooth muscle cells. They stain red with Masson trichrome and pink with PAS, but they fail to stain with phosphotungstic acid–hematoxylin, methyl green pyronine, Feulgen, Alcian blue at pH 2.5, or Congo red. Rarely, seminal vesicle fibrosis may be associated with retroperitoneal fibrosis and mediastinal fibrosis.123 Seminal vesiculitis—also referred to as male accessory gland infection, or MAGI124—is associated with infection and inflammation of adjacent organs, including the prostate, bladder, ejaculatory ducts, vas deferens, and epididymis.125 Compared with controls, patients with MAGI had higher levels of infertility, perhaps secondary to lower sperm progressive motility (11.4% versus 34.0%), higher percentage of abnormal forms (33% versus 9.0%), higher number of seminal white blood cells (2.2 versus 0.4/mL), and higher number of spermatozoa with DNA fragmentation (8.2% versus 1.0%).124 Acute vesiculitis is usually caused by retrograde infection with or without an indwelling catheter, ureteral or ejaculatory duct stenosis or anatomic anomaly, calculi, or surgical trauma. Studies in rats showed that the seminal vesicles are highly resistant to infection unless their secretory capability is decreased, as occurs with androgen deprivation.126 Antibiotic therapy is usually effective with the same agents as used for acute prostatitis; biopsies are rarely obtained in such cases and may be contraindicated because of complications of abscess formation and stricture. Protracted acute and chronic seminal vesiculitis results in atrophy and ejaculatory duct stricture. Abscess manifests with irritative voiding symptoms, fever, and pain in the scrotum, testis, perineum, or rectum; purulent ejaculation may also occur.127 Ultrasonography, computed tomography, and MRI are useful in verifying the diagnosis and directing transurethral incision and drainage.128,129 Chronic vesiculitis is associated with chronic prostatitis, and both disorders respond poorly to antibiotic therapy. Prior to the antibiotic era, the most common cause of vesiculitis was tuberculosis, which resulted in perineal fistula, fibrous adhesions, ejaculatory duct stricture, and massive circumferential calcification of the walls of the seminal vesicles at the site of previous necrotizing granulomas. Malakoplakia should be excluded.130 Seminal vesicle dilation and congestion may occur after prostatectomy, with resulting persistent dysuria.131 Schistosomiasis, usually secondary to Schistosoma haematobium infection of the bladder, involves the seminal vesicles more commonly than the prostate. Viruses (e.g., cytomegalovirus132), fungi, and parasites are rare causes of seminal vesiculitis. Rare cases of localized necrotizing vasculitis have been described.133,134 Bacillus Calmette-Guérin therapy may induce granulomas in the seminal vesicles that are similar to those in the bladder. Surgery for seminal vesiculitis is unnecessary unless the disorder is complicated by abscess, fistula, or stricture. In the early 1900s, the seminal vesicles were thought to be the cause of inflammatory rheumatoid disease, and perineal seminal vesiculotomy was popular at that time. This was also the treatment of choice for vesicular tuberculosis until the advent of antibiotic therapy. Success was reported with dilatation of the ejaculatory duct and flushing the seminal vesicle with an F9 seminal vesicle scope in patients with chronic and recurrent seminal vesiculitis.135 Calcification often follows seminal vesiculitis, particularly in patients with tuberculosis. Patients with a history of diabetes mellitus or uremia also develop dystrophic calcification of the seminal vesicles and other mesonephric derivatives. Most foci of calcification are idiopathic and asymptomatic, and imaging studies of the pelvis may detect them incidentally (Fig. 10-6). Calcification may be unilateral or bilateral and usually coexists with calcification of the vas deferens. Calcification is present within the muscular wall and often forms concentric rings; the mucosa is rarely involved. Osseous metaplasia is also rarely observed in the muscular wall. Suh and colleagues studied 298 consecutive whole-mount cases and found calcifications in 58.1% of seminal vesicles and 17.1% of ejaculatory ducts.136 Fig. 10-6 Idiopathic mural calcification of the seminal vesicle in a patient undergoing radical prostatoseminovesiculectomy for prostatic adenocarcinoma. Calculi are more frequent in the seminal vesicles than in the vas deferens, appearing as brown stones of variable number up to 1 cm in diameter (Fig. 10-7). They usually consist of phosphate and carbonate salts. The mechanism of formation is uncertain but may be caused by reflux of urine up the ejaculatory ducts.137,138 Cutaneous fistula is a rare complication that can be treated by fulguration and lithotripsy.139 An unusual case of bilateral large calcium oxalate monohydrate stones was reported in a 29-year-old patient with recurrent infections.140 Two cases of urothelial metaplasia of the seminal vesicles were reported as incidental findings in radical prostatectomy specimens.141 No evidence of prostatic involvement was found. Androgen deprivation therapy for prostate cancer can result in significant shrinkage of both the prostate and seminal vesicles that continues for at least 12 months.142 Seminal vesicle volume at 6 months decreased from 3.5 ± 1.8 to 1.9 ± 1.0 mL in one report. Radiation therapy for prostatic carcinoma causes atrophy and fibrosis of the seminal vesicles and perivesicular fat in 89% of patients.143 The golden-brown lipochrome pigment characteristic of the seminal vesicle epithelium is retained. MRI shows decreased luminal fluid and stromal fibrosis in approximately 37% of cases.144
Seminal vesicles
Embryology and anatomy
Age-associated changes
Congenital and acquired malformations
Cysts
Ectopic prostatic tissue
Nonneoplastic abnormalities
Amyloidosis
Stromal hyaline bodies
Fibrosis
Inflammation
Calcification and calculi
Urothelial metaplasia
Treatment changes (hormonal and radiation therapy)
Seminal vesicles