11 Sacral Neuromodulation
Introduction
Sacral neuromodulation (SNM) received approval from the U.S. Food and Drug Administration in 1997 (InterStim; Medtronic, Inc, Minneapolis, MN). SNM is indicated for the treatment of refractory urge urinary incontinence, frequency-urgency syndrome, and idiopathic urinary retention and chronic fecal incontinence. Although the exact mechanism of action has not been fully determined, SNM appears to modulate bladder behavior through electrical stimulation of somatic afferent axons in the spinal roots, which modulate voiding and continence reflex pathways in the central nervous system, likely by inhibiting interneuronal transmission in the bladder reflex pathway (Amend et al., 2011a). Neuromodulation also may have a direct effect on the pelvic floor.
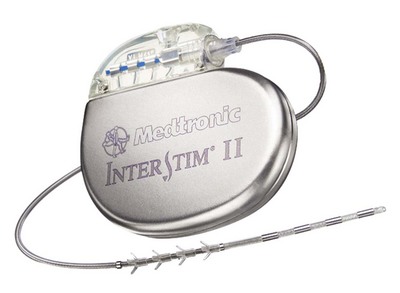
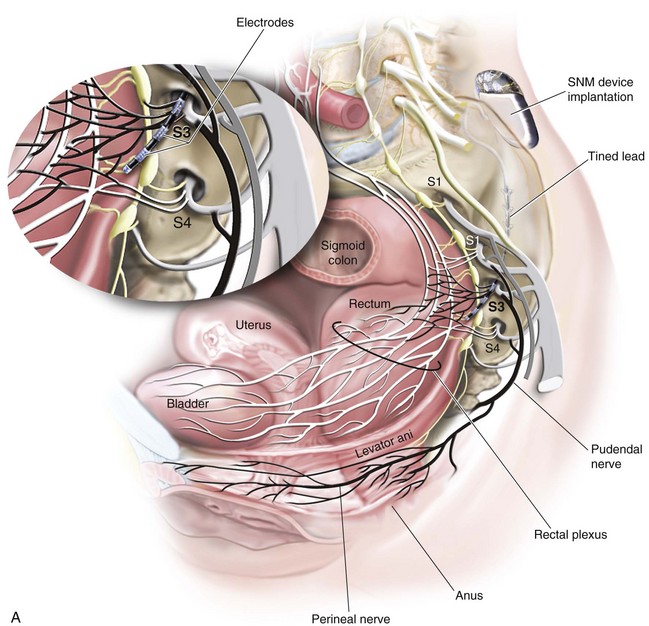
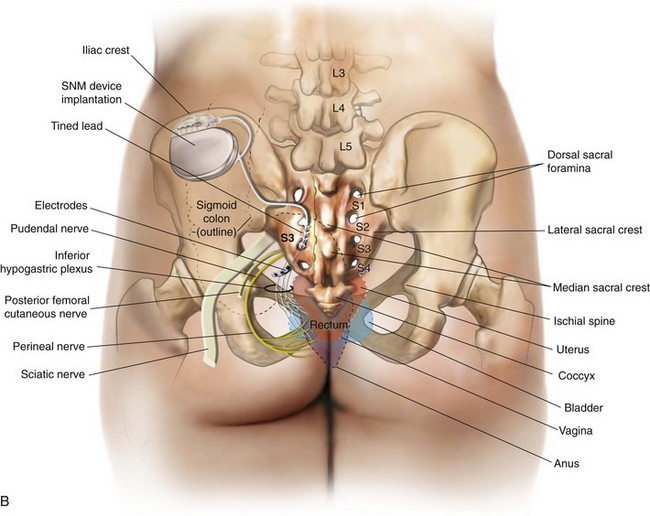
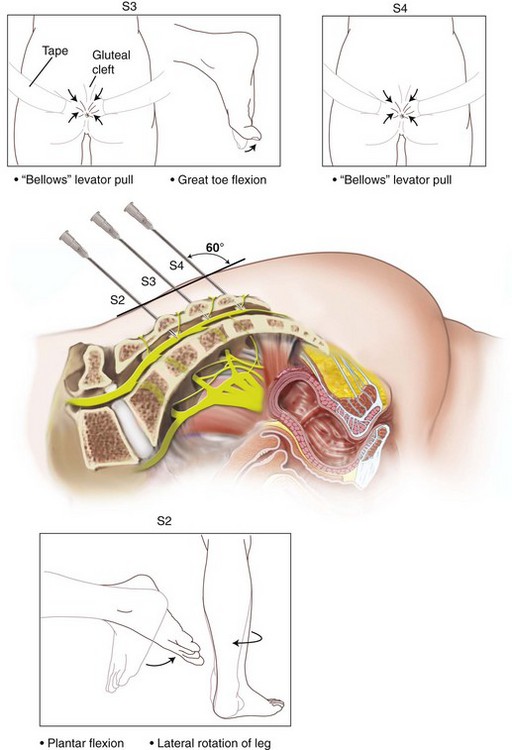
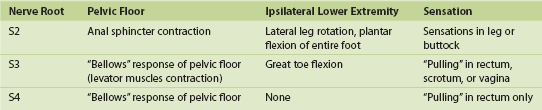
The InterStim device has three components: a battery-powered neurostimulator (also known as the implantable pulse generator [IPG]), an extension cable, and a tined electrical lead (Figure 11-1). The tined lead is a semipermanent, insulated electrical stimulation lead with four contact points near the tip (quadripolar) and four plastic collapsible projections (tines), which help to anchor the lead to the surrounding tissue. The IPG is a remotely programmable, battery-operated device that generates an electrical stimulus transferred to the lead contact points. With SNM, the electrical lead is implanted in close proximity to the third sacral nerve root (S3) at the level of the S3 spinal foramen (Figure 11-2). Stimulation of the S3 nerve root has demonstrated the best efficacy for SNM because S3 provides innervation to the bladder itself. Appropriate positioning of the electrical lead is verified by motor or sensory responses to electrical stimulation at the time of implantation (Figure 11-3). The S3 nerve root and neighboring S2 and S4 roots exhibit characteristic responses to electrical stimulation (Table 11-1). Ipsilateral great toe plantar flexion and pelvic floor “bellows” response are the predictable motor responses seen with S3 stimulation.
Sacral Neuromodulation Implantation Technique
Percutaneous or Peripheral Nerve Evaluation
1. Patient positioning, setup, and analgesia. Temporary lead placement during PNE may be performed in the outpatient clinic under local analgesia. The patient is positioned prone on the procedure table with slight flexion at the hips. The patient’s shoes and socks are also removed to allow observation of the feet. The nonsterile ground pad is affixed to the skin away from the site of lead placement (sterile field). The skin overlying the sacrum, buttocks, and perineum is sterilely cleansed, and surgical drapes are placed to allow visualization and inspection of the buttocks and gluteal crease. Analgesia is achieved through infiltration of the skin and subcutaneous tissues down to the bone in the vicinity of the desired sacral foramina with local anesthetic (e.g., lidocaine or bupivacaine).
2. S3 localization and lead placement. To locate the S3 foramen, anatomic landmarks or fluoroscopy may be used (Figure 11-4). When the approximate location of the S3 foramen is identified, a foramen needle is inserted percutaneously into the foramen at a 60-degree angle relative to the skin. The test stimulator is connected to the foramen needle via the test stimulation cable, and stimulation is applied. With electrical stimulation applied, the clinician observes for responses to stimulation (see Table 11-1 and Figure 11-3). If the desired responses are not seen, the needle may be moved up and down to change the depth, or a foramen needle may be placed on the contralateral side. If responses are consistent with different sacral nerves (i.e., S2 or S4), the needle may be reinserted into the appropriate foramen and retested. If the desired responses are seen, the test stimulation lead is inserted into the foramen needle until the lead electrode exits the needle tip (determined by markings on the lead). The lead is retested for appropriate responses, and if confirmed, the foramen needle is removed over the lead, leaving the lead in place in the S3 foramen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree