Robotic Surgery in Urology: Introduction
Minimally invasive surgery has played an important role in urologic surgery, with the widespread and frequent application of extracorporeal (shock wave lithotripsy), endoscopic, and laparoscopic techniques. Over the past decade, the introduction of robotic assistance has significantly altered the landscape of laparoscopic surgery in urology and will likely continue to play an important role in the future.
Background
Despite the variety and complexity of operations achievable with traditional laparoscopy, major limitations included the two-dimensional vision and instrumentation with restricted degrees of freedom as well as reduced tactile feedback (haptics). Thus, initial efforts were limited to extirpative or ablative procedures while technically demanding reconstruction requiring suturing and intracorporeal knot tying was primarily limited to highly experienced or specialized surgeons.
The concept of having an apparatus to aid or augment what is possible with the human hand is not new. The term “robot” is derived from the Czech word robota meaning “work” or “forced labor” and was introduced by the writer Karel Capek. Isaac Asimov coined the term robotics, although the definition of a robot is quite varied. Critical elements of a robot include programmability, flexibility, and ability to interact with the environment. Thus, most robots are computerized systems with mechanical capabilities.
With respect to surgical robots, there are those that are shared control (robot is primarily an assistant, such as camera holder), telesurgical, and supervisory controlled. The first robot utilized in urology was the PROBOT, an example of the last type where the robot performed transurethral resection of the prostate based on directions programmed into the controlling computer. The currently used robots are telesurgical or master–slave systems, wherein the surgeon controls the robotic arms during the procedure from a remote console and the robot is merely an instrument; thus, given the lack of automation, procedures performed with these machines may be better categorized as robotic-assisted surgery.
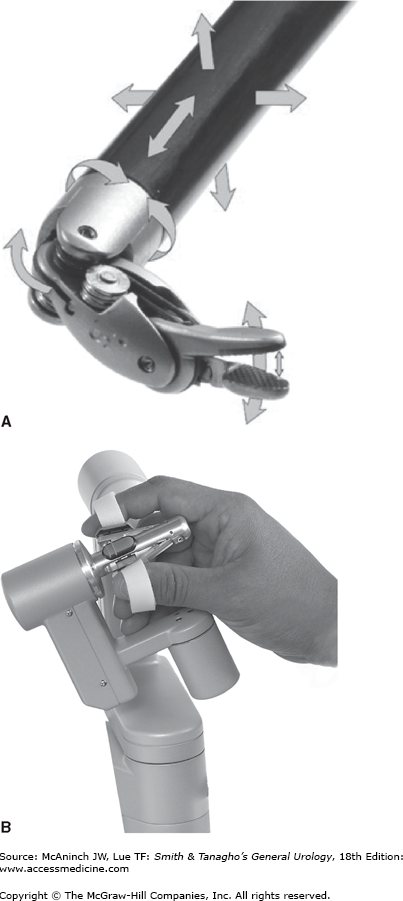
Computer Motion Inc. developed both AESOP, which controlled the endoscope using either voice or hand/foot control, and the ZEUS surgical system, consisting of three robotic arms attached to the operating table controlled remotely by the surgeon. In 2003, the company was acquired by Intuitive Surgical Inc., which was founded in 1995 and had developed the da Vinci surgical system, composed of a surgeon console, video tower and computer, and patient-side cart with three or four robotic arms.
Figure 10–1 shows the da Vinci system. It is currently the most widely utilized surgical robot with nearly 1500 units in operation worldwide. The surgeon sits at the console where an immersive, three-dimensional view of the surgical field is provided. Control of the instruments is achieved via free-moving finger controls, which translate the physical motion of the fingers and wrists into electrical signals and computerized such that the robotic arms mirror these movements (Figure 10–2). The surgical cart consists of either three or four arms, depending on the generation. True binocular vision is provided by a 12-mm scope with two separate video channels. A variety of inter-changeable instruments are available and attached to the robotic arms, which are introduced into the body through 8 mm or 5 mm ports. The ends of the instruments have seven degrees of freedom and are able to mimic the surgeon’s hand after tremor filtration and motion scaling, if desired. Unlike traditional laparoscopy, the da Vinci system incorporates (1) three-dimensional vision, (2) articulation of the instruments at the wrist, (3) improved surgeon ergonomics, and (4) natural translation of surgeon movements to instrument tips. It remains to be determined whether incorporation of robotics into traditional urologic procedures improves patient outcomes, but it is clear that for most surgeons, complex and reconstructive tasks are likely facilitated by robotic assistance. Although nearly every imaginable urologic operation, ranging from vasovasostomy to renal transplantation, has been performed with the aid of the robot, the remainder of the chapter will discuss the most common and well-established applications of robotic surgery within urology.
Lower Urinary Tract Operations
The initial target market for the da Vinci system was cardiothoracic surgery, attempting to minimize morbidity by avoiding a sternotomy and taking advantage of the precision and fine instrument movement afforded by the robot. Both coronary revascularization and mitral valve repair are currently performed with robotic assistance, but the operations are technically demanding and have not expanded as rapidly as the application to radical prostatectomy. The first laparoscopic radical prostatectomy was performed in 1991 and several centers reported their experiences with the minimally invasive approach in the late 1990s. Despite refinements in techniques and outcomes comparable with those of traditional open prostatectomy, laparoscopic radical prostatectomy remained a challenging operation, with significant learning curve, and was performed by relatively few surgeons. However, the operation ultimately proved to be the ideal application of robotic assistance and over the past decade, it has largely supplanted both open and laparoscopic prostatectomy; in 2010 it is estimated that 80% of radical prostatectomy operations in the United States will be performed with robotic assistance. Moreover the robot is widely available and has been rapidly adopted by surgeons throughout the country, in both community and academic medical centers.
The operation is fundamentally no different than open or laparoscopic radical prostatectomy, with the goals of complete removal of the prostate and seminal vesicles, performing lymphadenectomy when indicated, and preservation of urinary and sexual function. Various approaches have been described including transperitoneal versus extraperitoneal, and for the transperitoneal technique, the initial dissection can proceed either anteriorly (ie, retropubic space and through the bladder neck) or posteriorly (ie, dissection of the seminal vesicles and through the plane between the prostate and rectum). Regardless of technique, the patient must be placed in a steep Trendelenburg position in order to displace the bowels cranially, away from the pelvis. Some patients with underlying cardiac or pulmonary disease or obesity may not tolerate being in this position for a prolonged period. In addition, the abdomen is insufflated with carbon dioxide typically to 15 mm Hg pressure, further affecting pulmonary and cardiac function and requiring carefully monitoring by the anesthesia team. Meticulous attention to patient positioning is critical to prevent neuropraxia in the dark operating room environment; it may also be difficult to assess the patient with the additional equipment and bulk of the robotic system. Once the robot is docked in position with the camera and instruments inserted through the ports, the patient and operating room table cannot be moved until the instruments and robot are disengaged. Factors that make the robotic approach challenging include prior complex abdominal or pelvic surgery, morbid obesity, large prostate or median lobe, and prior radiation or prostate surgery; nevertheless, these are not absolute contraindications and surgery is often feasible despite these factors.
In assessing the success of robotic-assisted laparoscopic radical prostatectomy, the relevant outcomes of cancer control, urinary continence, and sexual function must be evaluated. Compared with open retropubic prostatectomy, robotic-assisted prostatectomy is associated with decreased risks of operative blood loss and need for transfusion.
With respect to oncologic outcomes, open radical retropubic prostatectomy is the gold standard against which new techniques must be compared. Large series of men undergoing surgery at high-volume centers have been published with long-term follow-up and include both pathologic outcomes and biochemical outcomes (Table 10–1). Smith et al (2007) reported lower rates of positive surgical margins in the robotic group compared with the open group (15% vs 35%, p< .001). Similar rates of positive surgical margins have been reported by Ahlering et al (2004) (16.7%), Menon et al (2007) (11%), and Patel et al (2010) (10.6%) and are likely not significantly different when compared with radical retropubic prostatectomy. In two large robotic series, the risk of positive margin was 4% and 13% in pT2 and 34% and 35% in pT3 (Badani et al, 2007; Patel et al, 2008). The risk of biochemical recurrence after robotic prostatectomy appears to be low and comparable with that of open prostatectomy. At median follow-up of 22 months, Badani et al (2007) reported 5-year actuarial biochemical-free survival of 84%. Longer follow-up is required to evaluate if there are significant differences in recurrence-free, and more importantly, cancer-specific survival. Using the SEER-Medicare Linked Database, Hu et al (2009) did not find a difference in rates of utilization of secondary cancer treatments, such as androgen deprivation and radiation therapy, for men undergoing minimally invasive versus open surgery, suggesting that cancer outcomes were similar.
Series | No. | OR time (mean) | EBL (mean) | Hospital stay (d) | Positive margins | Biochemical recurrence (%) | ||
|---|---|---|---|---|---|---|---|---|
pT2 (%) | pT3 (%) | Overall (%) | ||||||
60 | 231 | 103 | — | 9 | — | 16.7 | — | |
325 | 180 | 196 | — | 11 | — | 13 | — | |
100 | 208a | 141a | 1.1 | 9 | 50 | 15 | 16b,c | |
2766 | 154 | 142 | 1.14 | 13 | 35 | 12 | 7.3 | |
744 | 234 | 222 | 1.4 | 13 | 45 | 19 | 6.9 | |
1500 | 105 | 111 | 1 | 4 | 33 | 9 | 5 | |
400 | 186 | d | 3.1 | 10 | 42 | 19 | 13 | |
After open prostatectomy, continence rates 1 year after surgery are expected to be ≥90%. Most single institution series of robotic prostatectomy report comparable results, with ≥93% of men being continent 12 months after surgery (Table 10–2). Although there do not appear to be significant differences, men undergoing robotic prostatectomy may have more rapid return of continence. However, data from Hu et al (2009) suggest that men undergoing minimally invasive prostatectomy may have worse urinary outcomes compared with open prostatectomy. The population-based study found an increase in diagnosis of both incontinence and erectile dysfunction. It is important to note that the study did not differentiate between laparoscopic and robotic surgery, covered the early period of minimally invasive prostatectomy (2003–2007), and relied on Medicare claims rather than validated surveys to assess outcomes.
No. | Urinary continence rate (months) | Anastomotic stricture rate (%) | ||||
|---|---|---|---|---|---|---|
3 | 6 | 12 | >12 | |||
325 | 93 | 96 | — | — | 2.1 | |
500 | 89 | 95 | 97 | — | — | |
300 | 47 | 68 | 90 | 92 | 1.4 | |
1142 | — | — | 84 | — | — | |
395 | — | — | 91 | 95 | 3.8 | |
1100 | 85 | 96 | 97 | 98 | — | |
The enhanced three-dimensional and magnified visualization of the da Vinci system has led to a better appreciation and renewed consideration of the neuroanatomy of the prostate and parasympathetic innervation responsible for male erections. Techniques developed during the evolution of robotic prostatectomy to improve preservation of nerve bundles include high anterior release of the periprostatic fascia (coined the Veil of Aphrodite) and minimizing the use of thermal energy during dissection around the neurovascular bundles. Whether these maneuvers ultimately improve erectile function is unclear, but the reexamination of technique and anatomy has led to alterations in both minimally invasive and open surgical operations; it is difficult to determine which approach yields the best sexual function. Overall rates of potency after open prostatectomy have been reported to approach 70%, but are largely dependent on baseline function and age (Table 10–3). Approximately 60–70% of patients undergoing bilateral nerve-sparing robotic prostatectomy can be expected to have erections 12 months after surgery. As mentioned, Hu et al (2009) reported an increased diagnosis of erectile dysfunction (26.8 vs 19.2 per 100 person-years) in men undergoing minimally invasive prostatectomy. They also noted that the minimally invasive approach was associated with shorter hospitalization, lower rates of blood transfusion, fewer respiratory and surgical complications, and fewer anastomotic strictures. However, they noted an increased rate of genitourinary complications. Table 10–4 summarizes complications in published robotic prostatectomy series.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree