Fig. 24.1
Total robotic procedures performed worldwide (2005–2011). With permission from Intuitive Medical
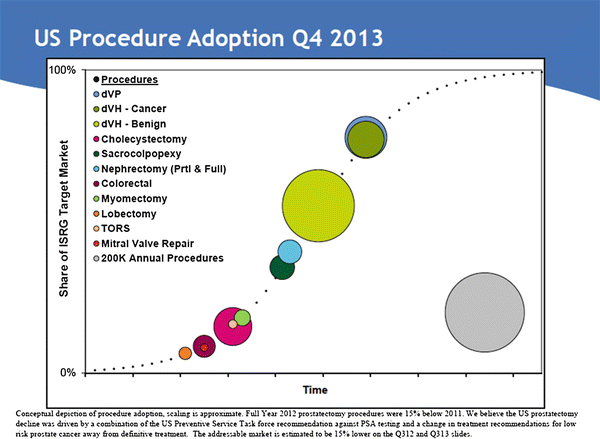
Fig. 24.2
Robotic cases by specific procedure. With permission from Intuitive Medical
This slow adoption is despite the many purported advantages of robotic surgery including better visualization, improved ergonomics, and overall dexterity. The robot provides a steady camera with highly magnified stereoscopic optics, which provides 3-D visualization and improved depth perception of the operative field. Furthermore, the surgeon at the console has complete control of the camera, removing any potential distraction of an assistant that normally used in traditional laparoscopic surgery. The superior surgical dexterity provided by the robotic approach is due to the instruments having seven degrees of freedom, 180° articulation, and 540° rotation—all allowing for easier manipulation within small spaces. The robot also allows for motion scaling and tremor filtering, which again facilitate technically challenging laparoscopic procedures. Moorthy et al. reported that the robot was associated with an enhanced dexterity by 65 %, reduction in skill-based errors by 93 %, and reduction in time needed to complete a task by 40 % [14]. The robot also allows for superior ergonomics [15], as the enhanced dexterity and superior visualization are especially helpful in the narrow confines of the pelvis, and is very appealing for surgical subspecialties that deal with pelvic pathology such as urologists, gynecologists, and colorectal surgeons.
The disadvantages of robotic-assisted surgery can be attributed to lack of haptic feedback, longer operative time, and cost. The time-consuming aspect of robotic surgery is docking, especially in certain totally robotic colorectal approaches which can require multiple docking and/or reengaging of instruments. However, it has been shown in rectal surgery that as experience is gained, operative time will improve [16]. Recent meta-analyses have suggested that operating time for robotic rectal procedures is similar to that for a conventional laparoscopic approach [12, 17]. The authors agree that with continued experience of an assembled robotic surgical team, the operative times of robotic colorectal procedures will approach the times of similar laparoscopic procedures. While there continues to be a debate regarding the cost-effectiveness of the robot, especially given the current lack of clinical evidence demonstrating its superiority compared to the laparoscopic approach, there is no doubt it has an expanding role for colorectal surgeons. In this chapter, we will review the technical aspects of robotic use for colorectal surgery and share several tips and tricks we have found useful in our experience with robotic approaches to colorectal disease.
Indications
The specific indications for the utilization of the robot when compared to laparoscopy continue to evolve. To date, there are no large randomized controlled studies that demonstrate the benefits of the robotic approach with regard to colorectal surgery. In fact, the majority of data detailing robotic use comes from single institution cases series. Therefore, the specific indications for colorectal surgery are evolving as new evidence comes to light. The clinical outcomes of robotic and laparoscopic colorectal procedures have overall been similar.
The utilization of robotics for colorectal resection is at the discretion of the surgeon. As mentioned above, the use of robotics can increase operative time, but once the learning curve is overcome, this is not a hindrance to the use of the robot. During segmental resections, the robot will allow for easier intracorporeal suturing, given the EndoWrist® technology. However, intracorporeal suturing has not translated into superior outcomes [18]. In short, despite the technological advantages afforded by the robot, and proven feasibility, there have been no tangible clinical improvements reported with the use of the robot for colon resections [13, 19, 20]. Rather, laparoscopic colon resection has the same clinical outcomes as robotic colon resection with a lower cost and shorter operative time.
Currently, however, there may be stronger indications for robotic rectal surgery given that studies have shown lower rate of conversion when robotic technology is used to facilitate total mesorectal excision (TME) [12, 13, 21]. The anatomical confines of the pelvis render rectal surgery more difficult compared to colon surgery, especially using a minimally invasive approach. Total mesorectal excision demands a high degree of precision, since anatomic dissection of the mesorectal envelope allows for the best oncological outcomes for rectal cancer. Moreover, the degree of difficulty for TME is directly proportional to the size of the pelvis [22]. Laparoscopic TME can be very challenging, especially in males and in those with very low tumors and obese patients [23]. Using the nonarticulating laparoscopic instrumentation and obtaining an optimal surgical view can become very challenging and lead to higher rates of conversion [24, 25]. The abovementioned advantages of the robot can overcome these challenges and, in fact, lead to lower conversion rates [12, 13].
We should point out that there are no absolute contraindications to the utilization of a robot in colorectal surgery—only the experience and expertise of the surgeon. Similar to laparoscopy, the robot is a tool or approach used to complete the same operation as in an open case. The robot can be used for diverticular disease, inflammatory bowel disease, and malignancy. The loss of haptic feedback may be more difficult in certain inflammatory conditions, and extra care must be taken when handling inflamed tissue. However, the indication should rely mostly on the underlying disease process; then consideration should be given to whether or not the patient can tolerate a pneumoperitoneum, steep Trendelenburg for pelvic cases, and perhaps longer operative times that occur with a robotic approach.
Equipment
The equipment needed for a laparoscopic case, as described by Dr. Bafford in Chap. 1, should be available for all robotic cases, especially when using the hybrid approach. Therefore, additional towers for insufflator, electrosurgical units, and extra monitors in rooms that are used for laparoscopic and robotic cases are necessary. The operating room setup should provide adequate space for staff and large equipment and allow the surgeon to have a direct view of the patient from the surgeon’s console. The room should also allow docking of the robot from several angles (Fig. 24.3).
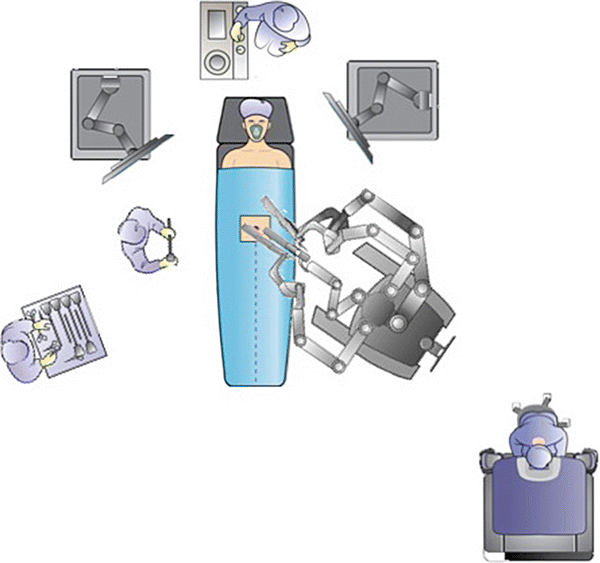

Fig. 24.3
Operating room setup should include ample room for robotic cart and laparoscopic and robotic towers. The system includes a robotic cart, console, and tower. With permission from Intuitive Medical
Robotic System
The da Vinci® exists in five models: standard, streamline (S), S-high definition (HD), and S-integrated (i) HD. At the time of this writing, the new da Vinci Xi has just been introduced into the market. This system features improved robotic arm movements and the ability to introduce the camera in any of the arms. The standard system originally was a three-arm robot. In 2006, the S-system offered numerous improvements including motorized patient cart, color-coded optic connection, easier instrument exchange, improved trocars, and increased range of motion and reach of instruments. The da Vinci® surgical system consists of three components: the surgeon’s console, the cart with the four robotic arms, and the electronic/vision tower (Fig. 24.3). The HD camera was an addition for the S-HD model, while the most recent version includes the enhanced HD vision at 1080i, upgrades to surgeon console and ability for dual console.
The surgeon operates at the console (Fig. 24.4); a three-dimensional image is obtained through the stereoviewer, which can be adjusted via the pod controls. The instruments are controlled using the master controllers and foot pedals. The surgeon’s instruments and console are only active when the surgeon’s head is at the stereoviewer. This allows for immediate deactivation of the surgical arms when the surgeon looks away from the surgical field.
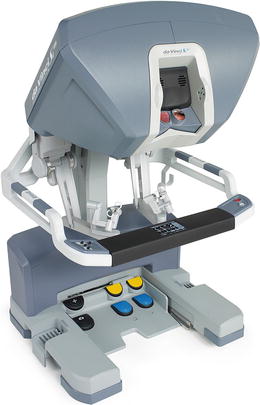

Fig. 24.4
The da Vinci Si™ console, which includes master controls and pedals. With permission from Intuitive Medical
The surgeon controls the instruments via the master controllers using the index finger and thumb. The robotic technology (without delay) scales, filters, and relays the information to the instruments. The surgeon should keep in mind the ergonomics while using the controllers and should be able to sit comfortably without overreaching. The robot is dual energy capable, and both monopolar and bipolar instruments can be used simultaneously.
The patient cart has 3–4 arms, one of which is the camera. Each arm has multiple clutch buttons for gross and fine movements (Fig. 24.5).
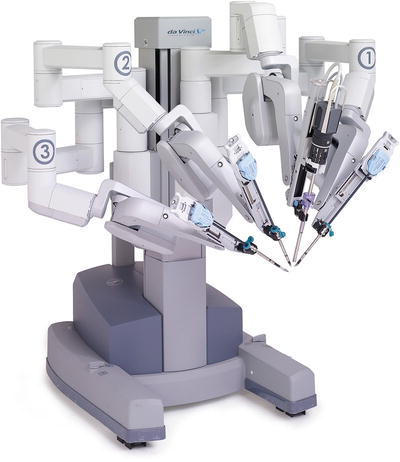

Fig. 24.5
Four-Arm da Vinci Si™ robotic cart. With permission from Intuitive Medical
Camera
The endoscope is available as a 0° and 30° lens. Our preference for robotic colectomy is to use a 30° lens, while we normally use a 0° scope for robotic TME. The camera system has digital zoom and allows for magnifications by pressing the left and right arrow keys on the left-side pod controls or depressing the camera pedal and moving the masters together or apart.
Instruments
As mentioned above, the EndoWrist® instruments allow for seven degrees of freedom, 180° of articulation, and 540° of rotation. Instruments are reusable but have a fixed number of uses, and the system keeps track of the number. The S-model instruments are 57 cm in length with blue housing and are made up of release levers, instrument shaft, wrist, and tip. Instruments come in a 5 mm “snake joint” (Fig. 24.6) or an 8 mm “angle joint” (Fig. 24.7). The snake joint uses a larger radius for rotation when compared to the angle joint. Our preferred instruments for colon resection and total mesorectal excision include prograsper in Arm 3, fenestrated bipolar forceps in Arm 2, and monopolar scissors/hook in Arm 1. Given the decreased haptic feedback, caution must be used when grasping the tissue. The mesorectum should not be grasped since this may cause tearing and bleeding.



Fig. 24.6
5 mm snake joint Maryland dissector. With permission from Intuitive Medical

Fig. 24.7
8 mm angle joint bipolar dissector. With permission from Intuitive Medical
Procedure-Specific Considerations
Positioning
Specific precautions as to patient positioning follow the same principles discussed in Chap. 2. However, we will reiterate that patients should be secured to the table so that they may not slip during the procedures. The use of shoulder and neck restraints is strongly discouraged, as these have been reported to cause brachial plexus injuries. The authors have successfully used a variety of methods including beanbags, gel pads, and the Pink Pad™, a proprietary specialized pad specifically made for patient positing during laparoscopic and robotic cases. For rectal cases, the patient should be positioned so that the operating room table and the robotic cart do not hinder access to the anus. The docking of the robot is procedure dependent.
Port Placement
In this chapter we will describe port placement that suits best the S/Si systems. It goes without saying that the new Xi platform will considerably change robotic approaches in a variety of operations and allow greater freedom in port placement. The setup described in this chapter have been optimized for single and two quadrant surgery in most cases; the new Xi system will surely make multi-quadrant robotic surgery much more feasible in the near future, but the experience with this device is still too limited at the time of this writing to describe it in detail. As in laparoscopic surgery, trocars are placed as to optimize triangulation, avoid collision within the body, and in the case of robotics avoid collision of the robotic arms outside the body. The principles of triangulation are maintained by assuring that minimum distance between trocars is at least one handbreadth apart. Ports should be placed under direct vision, and the small bowel swept out of the surgical field prior to docking of the robot.
We will describe port placement for each procedure below and will designate camera port (C), the three robotic trocars as R1, R2, and R3. Unless otherwise specified, Arm 1 will be docked in R1, Arm 2 in R2, and Arm 3 in R3. Arm 1 will usually be the operating arm and will be connected to a monopolar scissors, hook cautery, and/or robotic vessel sealer. Arm 2 and Arm 3 will provide traction and countertraction to aid in dissection. Arm 2 will often be connected to bipolar energy. A laparoscopic-assistant port can be helpful in further traction and/or suction/irrigation.
Right Colectomy
Positioning
The patient can be placed supine and/or in modified lithotomy position. Both arms should be tucked to allow standing room for the assistant and the cart. Once entrance into the abdomen is gained, and a laparoscopic view deems that patient anatomy is feasible to a minimally invasive approach, the table should be rotated to the left by approximately 20–30°. The robotic cart is docked at the patient’s right flank, and ports placed in the left abdomen as described below. The surgeon’s assistant will stand on the left side of the patient.
Port Placement
The camera port (C) is placed about two fingerbreadth to the left of midline, midway between the xiphoid and the pubis. R1 is an 8 mm trocar that is placed one handbreadth cephalad and lateral to C. R2 is an 8 mm trocar be placed one handbreadth inferior and medial to C. R3 is placed at a subxiphoid position. A 12 mm assistant port can be placed midway between C and the left anterior superior iliac spine (ASIS) and can be used for stapling, additional retraction, and passing sutures (Fig. 24.8).
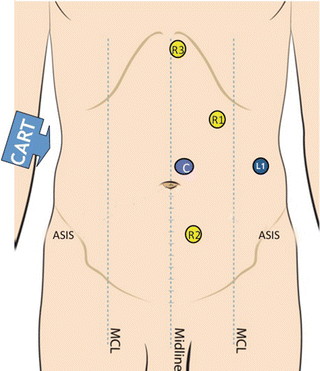

Fig. 24.8
Right colectomy port placement: C indicates camera port; L1 is a 12 mm laparoscopic assistant port. R1, R2, and R3 are 8 mm robotic ports. MCL midclavicular line, ASIS superior iliac spine. With permission from Intuitive Medical
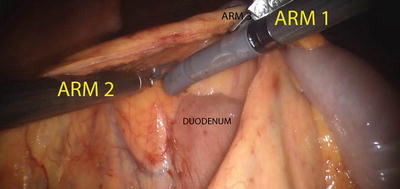
Procedure
Once the ports are placed and the patient is appropriately positioned, the robot is docked as described above. We prefer a medial-to-lateral approach for our colonic resection. The right colon is elevated and retracted anteriorly via Arm 3 (providing countertraction), and Arm 1 and Arm 2 are used for dissection and fine traction. The ileocolic pedicle is identified by the tenting of the mesentery and is isolated using monopolar cautery via Arm 1, while traction is provided via R2. The retroperitoneal structures, the duodenum and the head of the pancreas, are carefully swept posteriorly using Arm 1 and Arm 2 (Fig. 24.9). Once the pedicle is isolated, it is divided using the daVinci EndoWrist® One™ Vessel Sealer, clips, and/or an appropriate laparoscopic vessel sealing device or stapling devices inserted via the assistant port (Fig. 24.10). Our preferred method includes using Weck® Hem-o-lok® clips and vessel sealing device to divide the vasculature. Be sure to take the ileocolic pedicle at its origin for cancer cases. Once the ileocolic pedicle is divided, the dissection will proceed above the duodenum and head of the pancreas; the mesentery is gently stretched laterally, and the right branch of the middle colic artery is identified and divided. The terminal ileum and right colon are freed from their attachment to the pelvic sidewall and abdominal wall. Arm 3 will retract medially, while Arm 2 creates countertraction and Arm 1 takes down the line of Toldt via monopolar cautery. Once the bowel is fully mobilized, it can be extracted via a minilaparotomy for extracorporeal anastomosis. However, our preferred method is to create an intracorporeal anastomosis and extract via Pfannenstiel incision (Fig. 24.11). This is accomplished by aligning the terminal ileum and the transverse colon in an isoperistaltic fashion, creating an enterotomy and a colotomy, and firing a single 60 mm linear stapler cartridge to create the side-to-side anastomosis. The enterotomy can then be closed robotically in a running fashion using absorbable sutures (Fig. 24.12).