Retrograde Instrumentation of the Urinary Tract: Introduction
The ability to manipulate the urinary tract without the need for an open surgical incision differentiates urology from other disciplines. Such intervention may be required for diagnostic or therapeutic purposes (or both). Understanding the various catheters, guidewires, stents, endoscopes, and associated instrumentation is key in helping physicians accomplish their desired tasks. Manipulation of the urinary tract should be performed in a gentle fashion; instruments need not be forced. An understanding of anatomy and alternative instrumentation should allow physicians to accomplish their tasks with finesse. The patient should understand the proposed procedure and potential complications. For example, the attempt to place a retrograde ureteral catheter to drain an infected kidney may ultimately lead to a percutaneous nephrostomy if the surgeon is unable to achieve retrograde drainage. Knowing when to stop is as important as knowing when to start.
Many procedures are performed at the bedside or in a cystoscopy suite under local anesthesia. A patient who is comfortable, informed, and assured will more likely cooperate and tolerate the procedure. A physician who is familiar with the proposed instrumentation and understands its limitations and alternatives will win the patient’s confidence.
Manipulation of the urinary tract can result in significant injury. Anticipated prolonged procedures should be covered with appropriate antibiotics directed by preoperative urine cultures and sensitivities. Generous use of a water-soluble lubricant and low-pressure irrigation decreases the likelihood of significant iatrogenic infections. Patient positioning is as important as proper choice of instrumentation. Pressure points must be identified and adequately padded, especially when the patient is placed in the dorsal lithotomy position. In addition, the legs should be secured in their stirrups to prevent accidental injury, such as those that might result from a leg hitting the surgeon after an unexpected obturator reflex during endoelectric surgery.
Urethral Catheterization
Urethral catheterization is the most frequent retrograde manipulation performed on the urinary tract. Catheters are placed to drain the bladder during and after surgical procedures requiring anesthetics, to assess urinary output in critically ill patients, to collect reliable urine specimens, for urodynamic evaluation, for radiographic studies (eg, cystograms), and to assess residual urine. Such catheters can be left indwelling with a self-retaining balloon, as is done with a Foley catheter. An in-and-out procedure to drain a bladder does not require a self-retaining device. Adequate lubrication and sufficient frequency to keep the bladder at reasonable volumes are critical and must be emphasized to the patient performing self-intermittent catheterization; sterility is secondary. In contrast, when a catheter is left indwelling it is important to use sterile technique.
The penis should be positioned pointing toward the umbilicus to decrease the acute angulation as the catheter traverses the bulbar urethra. On most occasions, the catheter passes without difficulty. When difficulties arise, a careful history relating to previous urologic manipulations is critical. Strictures are not infrequent and can occur after endourologic surgery. Urethral strictures can be found from the meatus to the bladder neck. History of a straddle injury may suggest a bulbar urethral stricture. Adequate lubrication injected into the urethra and instruction of the patient to relax his pelvic floor eases the passage beyond the striated rhabdosphincter. A large-caliber catheter of approximately 18F should be used. Narrow, stiff, small catheters have greater potential of creating false passages and possible perforation. Coudé (elbowed) tipped catheters frequently help negotiate a high bladder neck, as seen with benign prostatic hyperplasia. With self-retaining Foley catheters, complete advancement until the elbowed valve is at the meatus or until the urine returns is important. Inflating the balloon prematurely (while it is in the urethra) may result in severe pain and possible urethral rupture. This must be emphasized to ancillary nursing personnel dealing with patients who are unable to communicate effectively, because under such circumstances, urethral rupture may present only after severe infection is evident.
It may be difficult to identify the meatus, especially in patients with obesity or hypospadias. Lateral and outward traction on the labia and the use of the posterior bill of a vaginal speculum may be helpful. With adequate instruction and a mirror to visualize the meatus, women can learn to catheterize themselves. For repeat catheterizations, a finger inserted into the vagina can help to guide the catheter.
When a urethral catheter cannot be placed, filiforms and followers may be used. The narrow filiform leaders are stiff and can puncture the urethra if too much force is used. Thus, gentle advancement should stop when resistance is encountered, and the initial filiform should be left in place. A second and third filiform, and possibly additional ones, should be placed next to the previously placed catheters in hope that the existing catheter occupies false passages or tortuous kinks. Eventually, one of the filiforms should pass and coil into the bladder. A screw adaptor at the end of the filiform can be used to connect progressively larger followers to dilate the narrowed urethra. After adequate dilatation, an open-tipped Council catheter can be placed over the filiform and into the bladder. If a problem or undue resistance is encountered at any stage, the procedure should be aborted and a suprapubic cystostomy should be placed to achieve adequate drainage.
Indwelling catheters should be secured to a closed gravity drainage system. Drainage tubing connected to catheters should be positioned to limit dependent curls and thereby limit airlocks that will frequently limit bladder evacuation. For long-term requirements in males, the catheter should be secured to the abdominal wall to decrease urethral traction pressure and potential stricture formation. Meatal care is needed to ensure adequate egress of urethral secretions.
Difficulty is much less common when removing indwelling urethral catheters. Here, the retention balloon is deflated prior to removal. On occasion, the balloon may not deflate. Inspection of the valve frequently reveals a problem. One may cut proximal to the valve in hopes of evacuating the balloon contents, but this is not always successful. Other options include transperineal or transabdominal balloon puncture (best with ultrasonographic guidance), or injection of an organic agent such as ether through the balloon port (with a full bladder to prevent chemical cystitis) to dissolve the balloon wall. If the catheter cannot be advanced, retracted, or twisted, one should suspect an unintended suture that could have been placed during prior surgery; such sutures can be cut via the small pediatric endoscope placed along the Foley catheter. Another complication of urethral catheters is incrustation, especially when a catheter is left indwelling for a long time.
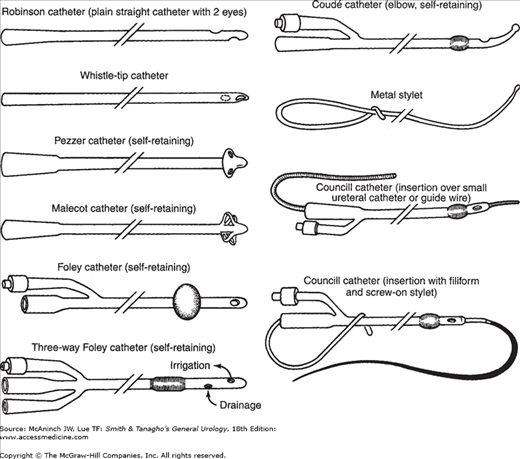
Catheters differ in size, shape, type of material, number of lumens, and type of retaining mechanism (Figure 11–1). Standard sizes of external catheter diameters and most endoscopic instruments are given according to Charriére’s French scale (units of 0.33 mm = 1 French [F] or 1 Charriére [Charr]). Thus, 3F equals 1 mm in diameter and 30F equals 10 mm in diameter.
The choice of catheter size is dependent on the patient and the purpose. Large-caliber catheters are used to evacuate blood clots or other debris. Other catheters are used to stabilize grafts after open urethroplasties, for stenting after endoscopic incisions of strictures, for support of external ureteral catheters, or to assess urinary output. Triple-lumen catheters (one port for balloon inflation and deflation, and one each for inflow and outflow) have smaller lumens than two-way catheters. Other catheter variables include balloon size and construction materials; smaller catheters typically have smaller balloons. Large balloons (eg, 30 mL) can be inflated well over 50 mL to decrease the likelihood of the balloon migrating into the prostatic fossa, especially after transurethral resection of the prostate (TURP). They can be used as traction devices against the bladder neck to control hemorrhage from the prostatic fossa after TURP.
The rigidity of the catheter, the ratio between internal and external diameters, and the biocompatibility depend on the material with which the catheter is made. The standard latex catheter can result in severe reactions in patients with latex allergies, most commonly seen in those with myelomeningoceles. Silicone varieties are good alternatives in such situations. Mucosal irritation is decreased when catheters with a low coefficient of friction are used. Hydromers are placed onto catheters to allow for transient coating, creating an interface between biologic tissues and the foreign catheter; this interface lasts for approximately 5 days. Permanent hydrogel coatings last the life of the catheter. Decreasing the coefficient of friction of these catheters brings about a decrease in mucosal irritation and better biocompatibility. Catheters with a longer lasting interface result in decreased incrustation.
Urethroscopy
To identify and aid in treating urethral pathology, endoscopic inspection via a urethroscope with a 0° lens is helpful. Stricture disease can be identified or confirmed after radiographic studies. Strictures are characterized by circumferential narrowing. Sequential dilation of urethral strictures by inserting catheters of increasing size exerts shear and tear forces to the mucosa and is likely to produce extended scarring. Thus, stricture recurrence is common if periodic urethral dilation is terminated. Balloon dilation of a stricture with 7–9F balloon dilators (which can be passed over guidewires and inflated up to 30F with pressures of up to 15 atm) does not exert shear force, but the long-term results are varied. Limited circumferential strictures can be incised under direct vision with an endoscopic cold knife. The incision is usually made at the 12-o’clock position, adequate to allow passage of the urethroscope. The bladder then can be evacuated and adequate irrigation used if further incision results in hemorrhage. It is difficult to identify the true extent and depth of a stricture solely by vision because scarring can involve deeper tissues. Here, urethral ultrasonography is an adjunct.
Urethral diverticulum can be identified with urethroscopy. A catheter can be placed through the neck of the diverticulum to help confirm its location during definitive open surgical repair. Urethroscopy can be used to direct injection of dye into rare retained müllerian duct cysts, to identify and extract foreign bodies or rare calculi, and to access biopsy-suspicious lesions. Urethroscopy allows endoscopic treatment of urethral condylomata.
Cystoscopy
Endoscopic inspection of the lower urinary tract requires irrigation, illumination (fiberoptics), and optics. The optics and illumination are offset by the irrigating and working port. To optimize a complete examination, the rigid endoscope should be rotated, and 0°, 30°, 70°, and 120° lenses may be required. Suprapubic pressure facilitates inspection of the bladder dome, which frequently has an air bubble. A systematic approach is required when evaluating the urethra, prostate, bladder walls, dome and neck, and ureteral orifices (including location, number, shape, and character of efflux). The bladder should be evaluated at different levels of filling. It is only after full distention of the bladder that characteristic glomerulations and ecchymoses are seen in patients with interstitial cystitis. Rectal examination with the endoscope in place is informative, especially in assessing prostate size and length of prostatic urethra. Similarly, concurrent vaginal examination in women can be useful in evaluation of cystoceles.
Choice of irrigant during endoscopic manipulation is important. There are conductive and nonconductive irrigants. Conductive irrigants, which include saline and lactated Ringer’s solution, would be inappropriate during traditional endoelectric surgery because the electrical charge would be diffused by the irrigant. Nonconductive irrigants include water and glycine. Water has a theoretic advantage of increasing visibility, and because it is hypotonic, it can lyse tumor cells. If the potential exists for increased intravascular absorption, iso-osmotic or other nonhemolyzing agents are preferred to hypotonic solutions.
Rigid endoscopy results in discomfort, which can be minimized with 1% lidocaine per urethra as a local anesthetic. Flexible endoscopes decrease patient discomfort and allow for instrumentation in the supine rather than the dorsal lithotomy position. They are now used routinely in an office setting for hematuria/tumor surveillance and double-J stent retrieval. Videoendoscopy with flexible scopes allows patients to visualize normal and abnormal anatomy and thus helps them understand their pathology. Videoendoscopy reduces fluid contact to the urologist and can help reduce potential cervical neck disease exacerbated with altered posture when endoscopy is performed without videoendoscopic monitoring. However, there are disadvantages. Flexible scopes have smaller irrigating ports, and they do not have a working sheath. As a result, changing lenses, assessing residual urine, and repeat evacuation of irrigant cannot be completed without entirely removing the endoscope. Rigid endoscopy allows for a greater variety of instrumentation, better optics, and increased durability.
Instrumentation similar to that used to evaluate the urethra and bladder can be used to inspect continent urinary reservoirs or conventional ileal loops. A Robinson or a Foley catheter placed prior to the endoscope gives the operator a visual landmark and an exit port for irrigation to keep the procedure at a low pressure. Alternatively, the Foley balloon can be inflated and the catheter plugged to transiently expand the intestinal segment in hopes of helping to identify landmarks or pathologic lesions. Endoscopic inspection allows for identification of calculi, foreign bodies and mucous plugs, and also has the potential for intubation of ureterointestinal anastomoses.
Ureteral Catheterization
Ureteral catheterization is required in performing retrograde pyelography, collecting urine for cytologic examination or cultures, and performing brush biopsies (Figure 11–2). Other procedures (Figure 11–3) that require ureteral catheterization include draining an obstructed kidney due to either intrinsic or extrinsic compressions and placement of an internal double-J stent. Finding the ureteral orifice can be difficult. Long-term indwelling Foley catheters, infection, history of ureteral reimplantation, or renal transplantation can hinder identification of the ureteral orifice. One must first try to identify the interureteric ridge and then look for a jet of urinary efflux. Varying bladder volumes and use of intravenous methylene blue may be helpful. However, it may take up to 5–20 minutes for intravenous agents to be excreted out of the ureteral orifice. Once the orifice is identified, catheters usually are placed uneventfully. However, in the setting of benign prostatic hyperplasia with J-hooking of the distal ureter, previous retroperitoneal surgery, reimplantation of the ureter, decreased lower extremity mobility or other skeletal abnormalities, or edema or kinking secondary to long-standing impacted ureteral calculi, catheterization procedures can be difficult or impossible. An Albiron bridge may help direct catheters and guidewires.
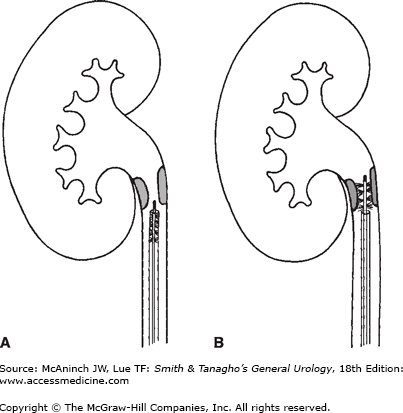
Figure 11–2.
Brushing of a proximal ureteral lesion. A:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree