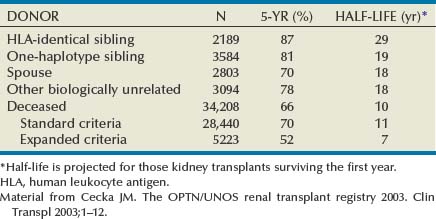
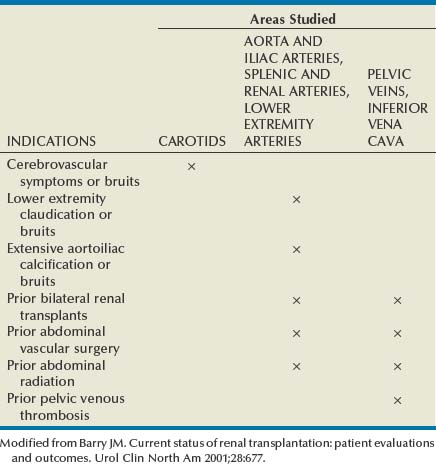
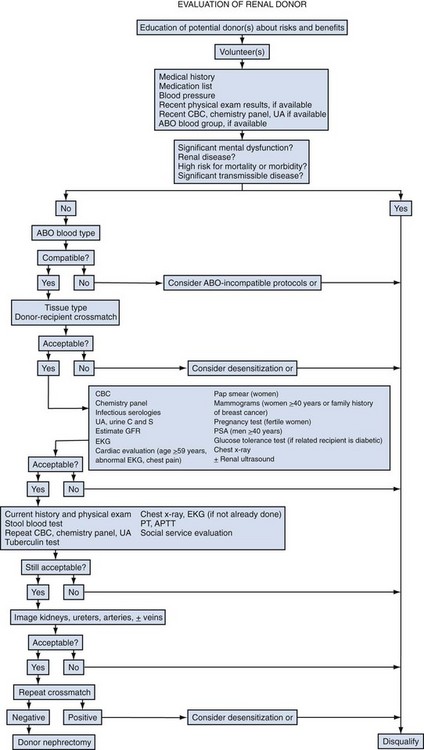
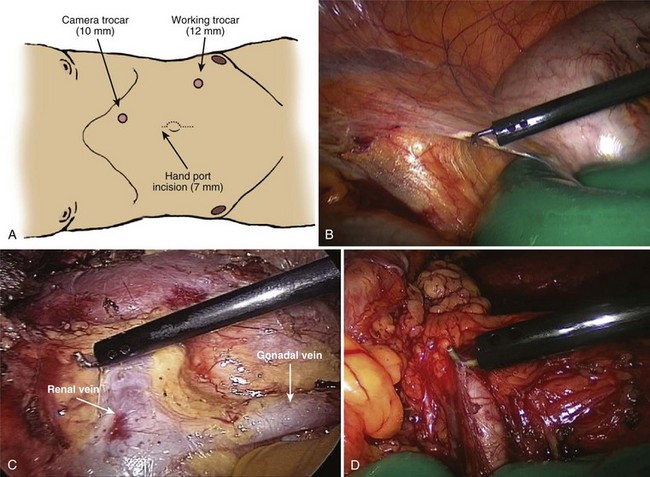
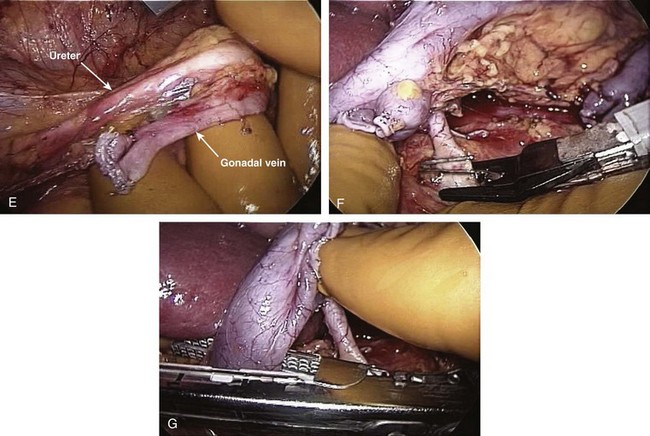
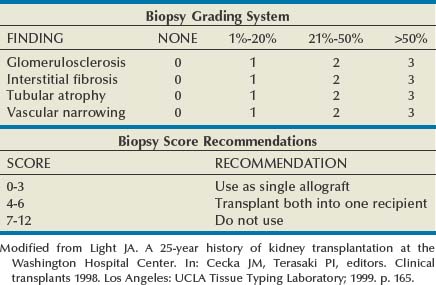
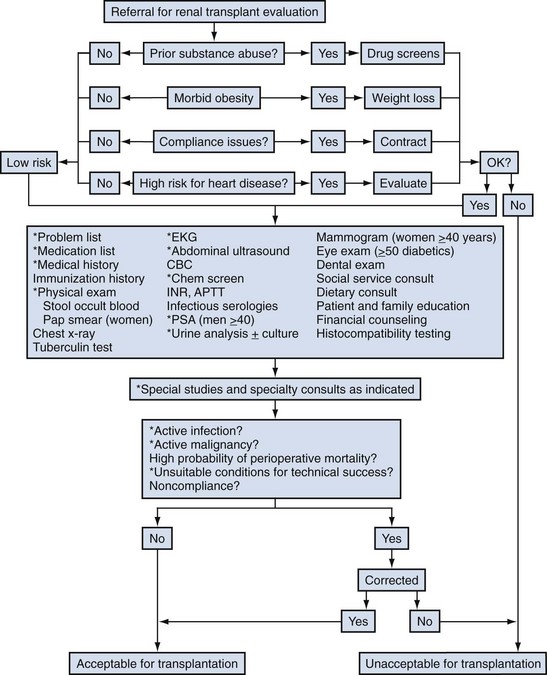
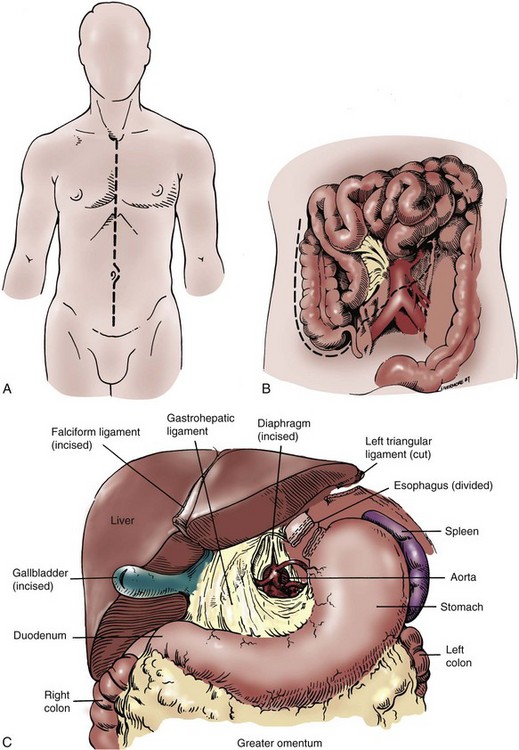
John Maynard Barry, MD, Michael Joseph Conlin, MD, FACS Kidney transplantation is the pioneer discipline in solid organ transplantation, and the relationship between transplant surgeon and nephrologist has served as a model for multidisciplinary team care. The Scientific Registry of Transplant Recipients (SRTR) provides a concept of national and center-specific outcomes data that can be applied to other disciplines in urology. The estimated number of patients starting renal replacement therapy each year for end-stage renal disease (ESRD) in the United States is about 360 per million population (U.S. Renal Data System [USRDS], 2010). The median age of these new ESRD patients is 64 years. Both the prevalence and the incidence of ESRD are more common in elderly than in young patients, in men than in women, and in African-Americans, Hispanics, and Native Americans than in Asians and whites. Diabetes mellitus is the most frequent cause of ESRD, followed in order by hypertension, glomerulonephritis, and renal cystic disease. Diabetes is especially common among Native Americans, and hypertension is disproportionately high among African-Americans. The incidence of ESRD is greater than that for any urologic malignancy except prostate cancer, and more patients die annually from ESRD than from any urologic malignancy (Heron et al, 2009; Jemal et al, 2009; USRDS, 2010). The purposes of renal replacement therapy are to prolong and maintain the quality of life. The treatment or treatments that are chosen for an individual patient are those that will allow the longest extension of useful life. Renal replacement therapy is now considered to be a right in the United States and most other developed countries. Permanent renal failure in adults is commonly defined as an irreversible glomerular filtration rate (GFR) of less than 10 mL/min or a serum creatinine level of greater than 8 mg/dL (Annual Report, 1994). About half of the patients who initiate renal replacement therapy have an estimated GFR less than or equal to 10 mL/min (USRDS, 2010). Patients with symptomatic uremia, especially children and diabetic adults, are considered on an individual basis as meeting criteria for permanent renal failure The generally accepted indications for dialysis initiation are pulmonary edema resistant to diuresis, severe uremic symptoms, hyperkalemia that fails to respond to medical therapy, metabolic acidosis unresponsive to medical therapy, or uremic pericardial effusion. A reasonable goal for ESRD treatment programs is the transplantation of all patients in whom the risk is equal to or less than that of remaining on maintenance dialysis. Renal transplantation is the preferred method of therapy for most patients with ESRD because it is more cost effective and allows a return to a more normal lifestyle than maintenance dialysis does. A recent quality of life year (QALY) cost analysis strongly favored transplantation over dialysis (Mendeloff et al, 2004). The number of patients listed for kidney transplantation continues to expand disproportionately to the number of kidney transplantations performed annually. There are currently more than 80,000 patients waiting for deceased donor kidney transplants, and with about 10,000 deceased donor kidney transplants performed annually, there are eight times as many ESRD patients waiting as there will be deceased donor kidneys available in the coming year. Despite this obvious disparity, the number of deceased donor kidney transplants has increased only 15% over the past 10 years, and this is mostly due to the increased use of kidneys from expanded-criteria donors (ECDs) and donation-after-cardiac-death donors (DCDs) (Scientific Registry of Transplant Recipients, 2009). The inadequate supply of deceased donor kidneys is one of the factors that has influenced the increase in the number of living donor kidney transplants in the past decade. The increase in living renal donation has been further facilitated by the widespread adoption of minimally invasive donor nephrectomy techniques; the increased use of living, biologically unrelated renal donors; and the development of protocols for transplantation across alloantibody barriers including ABO blood group incompatibility. Xenotransplantation and tissue engineering are fields of research for the continuing problem of inadequate supply of transplantable organs. Hemodialysis is the predominant form of therapy for adults with ESRD. In the United States, it accounts for about two thirds of the treated ESRD patients (USRDS, 2010). Transplantation, however, is the predominant mode of care for patients younger than 20 years. About 10% of ESRD patients are treated with chronic peritoneal dialysis. A desire for self-care, a long distance from a hemodialysis unit, difficulties with hemodialysis therapy, serious cardiac disease, diabetes mellitus, and small stature are characteristics of patients especially suited for peritoneal dialysis. Unsuitable characteristics of patients for chronic peritoneal dialysis are considered to be obesity, hernias, poor hygiene, inflammatory bowel disease, and obliterated peritoneal space (Nicholson, 2001). When compared with adults with ESRD, children are more likely to receive chronic peritoneal dialysis, and they are more likely to undergo renal transplantation (USRDS, 2010). There is a relatively greater availability of parental kidney donors, and children receive preferential points when they are listed for deceased kidney transplantation. Special problems in children with ESRD include growth failure, poor nutrition, and psychiatric problems. They often suffer from a cycle of depression, anxiety, and loss of self-esteem, and these problems can result in family stress and divorce (National Institutes of Health [NIH] Consensus Development Conference, 1993). The development of recombinant growth hormone has made possible the treatment of short stature in pediatric patients with chronic renal failure and after renal transplantation. Nutritional supplementation can be done by nasogastric feeding tube or button gastrostomy. Data from the USRDS indicate that survival after renal transplantation is significantly better than that of patients treated with dialysis (USRDS, 2010). Although this may simply mean that healthier patients are more likely to be transplanted, more controlled analyses have indicated a significantly reduced mortality risk for renal transplant recipients when compared with acceptable transplantation candidates waiting on dialysis (Meier-Kriesche et al, 2001). Regardless of whether the treatment modality is dialysis or transplantation, the major causes of death are, in order, heart disease, sepsis, and stroke (USRDS, 2010). The survival of kidney grafts has improved steadily over the past 3 decades but is now at a plateau. The results for the current era are quite remarkable (Table 44–1). The history of renal transplantation illustrates the successful combination of the fields of surgery, medicine, immunology, and government. Carrel established the modern method of vascular suturing at the turn of the 20th century, and he was awarded the Nobel Prize in 1912 for his work on organ grafting (Hamilton, 2001). In 1933 the first human renal allograft was performed by Voronoy in the Ukraine (Hamilton and Reid, 1984). The recipient was a 26-year-old woman who had attempted suicide by ingesting mercuric chloride. The donor was a 66-year-old man whose kidney was removed 6 hours after death. Under local anesthesia, the renal vessels were anastomosed to the femoral vessels and a cutaneous ureterostomy was performed. A small amount of blood-stained urine appeared, but the patient expired 48 hours after the procedure. The first long-term success with human renal allografting, in which the patient survived for over a year, occurred in Boston in 1954, when a kidney from one twin was transplanted into the other, who had ESRD (Murray et al, 1955). In 1958 the first histocompatibility antigen was described. Radiation was used for immunosuppression in 1959, azathioprine became available for human use in 1951, and glucocorticoids became part of a standard immunosuppression regimen with azathioprine in 1962 (Goodwin et al, 1963). In the same year, the first use of tissue matching to select donor-recipient pairs was done. The direct crossmatch between donor lymphocytes and recipient serum was introduced in 1966, and heterologous antilymphocyte serum was used as an immunosuppressant in human renal transplantation. In the late 1960s human renal preservation over 24 hours became possible with either pulsatile machine perfusion (Belzer et al, 1967) or simple cold storage after flushing with an ice-cold intracellular electrolyte solution (Collins et al, 1969). The beneficial effect of blood transfusions was described by Opelz and colleagues in 1973, and this led to immunologic conditioning with blood products for both deceased and living donor renal transplants. Donor-specific blood transfusions eventually became part of standardized pretransplantation immunologic conditioning protocols for living donor renal transplantation (Salvatierra et al, 1980). In the cyclosporin era, the beneficial effects of random blood transfusions and donor-specific blood transfusion protocols have been difficult to establish, and transfusion protocols have been associated with donor-specific sensitization and the transmission of viral illnesses. For these reasons, pretransplantation transfusion protocols are no longer routinely prescribed. Medicare coinsurance for ESRD patients was passed into law in 1972 and instituted in 1973. This removed a significant impediment to renal transplantation in the United States. In the mid-1970s, brain-death laws were passed. This allowed organ retrieval from beating-heart deceased donors, reduced warm ischemia time, and improved the quality of deceased donor kidney grafts. The first clinical trials of cyclosporine were reported by Calne and colleagues in 1978, and 3 years later there were reports of the successful use of a monoclonal antibody for the treatment of renal allograft rejection in humans (Cosimi et al, 1981). In 1984 Congress passed the National Transplant Act, which authorized a national organ-sharing system and grants for organ procurement. The University of Wisconsin (UW) solution, introduced in the late 1980s, provided one solution for the preservation of all transplantable abdominal organs including kidneys (Belzer and Southard, 1988). Recombinant erythropoietin became available in 1989, and this significantly improved the quality of life for maintenance dialysis patients and reduced the need for blood transfusions (Carpenter, 1990). This has decreased the risk of virally transmitted infections and the risk of the development of anti-human leukocyte antigen (HLA) antibodies in potential kidney transplant recipients. Thirty-six years after the first long-term success of human-to-human kidney transplantation, Joseph E. Murray received the Nobel Prize in Medicine in 1990 for his pioneering work in renal transplantation. Laparoscopic donor nephrectomy was introduced in 1995 to reduce disincentives for living donor nephrectomy (Schulam et al, 1996; Pradel et al, 2003). The pretransplantation evaluation is a multidisciplinary process that is performed well in advance of the renal transplantation operation and immunosuppression. The purposes of the evaluation are generally considered to be to diagnose the primary renal disease and its risk of recurrence in the kidney graft and to rule out active invasive infection, a high probability of operative mortality, noncompliance, active malignancy, and unsuitable conditions for technical success (Barry, 2001; Kasiske et al, 2001; Bunnapradist and Danovich, 2007). The process of evaluating the transplantation candidate is initiated by identifying the presence or absence of the risk factors at the top of the algorithm in Figure 44–1. Candidates with a history of substance abuse must have unannounced drug screens with negative results before continuing the process. Candidates with morbid obesity, defined as a body mass index of greater than 35, must demonstrate weight reduction before continuing the process because of the perioperative risks and earlier graft failures associated with renal transplantation in obese patients (Glanton et al, 2003; Meier-Kriesche et al, 2003). Bariatric surgery may be an option to prepare dialysis-dependent morbidly obese patients for transplantation (Alexander et al, 2004; Modanlou et al, 2009). Candidates considered to be at risk for noncompliance must demonstrate contract satisfaction. For example, a hemodialysis-dependent patient may be required to maintain his or her serum phosphorus level less than or equal to 6 mg/dL, predialysis serum potassium level less than or equal to 6 mmol/L, and interdialytic weight gain less than or equal to 5% of dry weight for a period of 3 months. It is recommended that patients older than 50 years or with a history of coronary artery disease, cardiac symptoms, or insulin-dependent diabetes mellitus undergo cardiac testing with further diagnosis and treatment of significant cardiac disease before proceeding with the rest of the algorithm (Lewis et al, 2002). (Modified from Barry JM. Current status of renal transplantation: patient evaluations and outcomes. Urol Clin North Am 2001;28:788.) Patients with focal segmental glomerulosclerosis, hemolytic-uremic syndrome, and primary oxalosis should be counseled about the significant probability of disease recurrence and the risk of secondary graft failure (Cameron, 1993; First and Peddi, 1999; Hariharan et al, 1999; Jamieson, 2005). Patients with a high risk of recurrent focal segmental glomerulosclerosis are those younger than 15 years of age, those who have a rapidly progressive course, and those with mesangial proliferation on biopsy of the native kidneys. If a first transplant has failed because of recurrent focal segmental glomerulosclerosis, the risk of recurrence in a second transplant approaches 80%. Hemolytic-uremic syndrome is a relatively common cause of ESRD in children, and recurrence has been associated with calcineurin inhibitor immunosuppression. Oxalosis can rapidly recur in a kidney graft, and this disease is probably best treated with combined liver and kidney transplantation to correct both the metabolic defect and the renal failure. The ESRD of renal amyloidosis, cystinosis, and Fabry disease are all potentially treatable with renal transplantation despite significant recurrence rates. Infections must be detected and treated before transplantation or prevented with immunizations. Potential sources of dental sepsis must be treated. Dialysis access sites must be clear of infection. Pulmonary infections or tuberculin test conversions require treatment before immunosuppression. Reasonable indications for pretransplantation cholecystectomy are considered to be symptomatic cholecystitis and cholelithiasis with gallbladder wall thickening. Segmental colectomy is reasonable for patients with recurrent diverticulitis because of the morbidity and mortality of bowel perforation in the immunosuppressed kidney transplant recipient (Salem et al, 2004). Diabetic foot ulcers must be healed before transplantation, and urinary tract infection should be inactive at the time of engraftment. Serologic testing for cytomegalovirus (CMV) is important because this disease is a major cause of morbidity in immunosuppressed patients. Patients who are seropositive for herpes simplex virus or who have a history of oral or genital herpes simplex or shingles need antiviral therapy during intense immunosuppression. Epstein-Barr virus titers are determined in children and, for the Epstein-Barr virus–seronegative child, a kidney from an Epstein-Barr virus–seronegative donor is preferred to reduce the risk of a post-transplantation lymphoproliferative disorder, the commonest new malignancy in pediatric organ transplant recipients. Recipient human immunodeficiency virus (HIV) infection has been considered to be a contraindication to renal transplantation; however, successful outcomes have been reported in HIV-positive patients who have had low viral loads and sufficient CD4 T-cell counts. Chronic active hepatitis is a major cause of mortality in the late post-transplantation period. The treatment of chronic, active hepatitis B and C is evolving, and patients with active hepatitis C infection may benefit from antiviral therapy before transplantation (Mahmoud et al, 2005). To reduce the risk of cancer recurrence, a waiting time of 2 to 5 cancer-free years from the time of the last cancer treatment has been recommended for patients who have had invasive malignancies (Kasiske et al, 2001). Shorter intervals from cancer treatment to transplantation are generally accepted for patients who have had low-grade, noninvasive cancers. The calculation of expected remaining life times after transplantation (USRDS, 2010) and the application of cancer progression nomograms to recommend cancer-free intervals before transplantation may be helpful in individual cases (Secin et al, 2004). Cholecystectomy is recommended for transplantation candidates with gallbladder polyps greater than 1 cm in diameter because of the risk of malignancy (Boulton and Adams, 1997; Chattopadhyay et al, 2005). Heart disease is the predominant cause of death after renal transplantation (USRDS, 2010), and it is common for kidney transplantation candidates with a history of cardiac disease, cerebrovascular disease, or diabetes mellitus or who are older than 50 years to undergo a cardiac performance evaluation. Further testing and treatment are based on the results of a screening evaluation. Cerebrovascular disease, peptic ulcer disease, and significant pulmonary disease must be detected and treated. For example, patients with autosomal dominant polycystic kidney disease (ADPKD) and headaches or a family history of stroke should be screened for cerebral aneurysms (Pirson et al, 2002). Cigarette smoking increases the risks of surgery, post-transplantation malignancy, cardiovascular disease, and renal allograft loss (Sung et al, 2001). It must be stopped before transplantation in patients who already have clinical evidence of vasculopathy or cardiopulmonary disease. Compliance issues are extremely important in the long-term management of kidney transplant recipients. Transplantation candidates with chemical dependency need to have an objectively documented drug- or alcohol-free period of at least 6 months before transplantation (Kasiske et al, 2001). Financial and psychosocial consultations are necessary to identify and correct problems that could result in noncompliance, for example, the lack of funds to pay for maintenance immunosuppressants or a mental handicap that could prevent the recipient from following a post-transplantation treatment plan. Patients with symptoms and signs of lower extremity arterial disease or a history of abdominal or pelvic vascular surgery need to undergo a diagnostic evaluation to be certain that revascularization of a kidney graft is possible. Vascular screening with Doppler flow studies is recommended for the indications listed in Table 44–2. If significant arteriosclerosis or venous disease is detected, it may be necessary to perform angiography on select alternative sites such as the aorta or splenic artery for renal revascularization or to plan corrective vascular surgery before renal transplantation. Thrombosis is a significant cause of kidney transplant loss, especially in children (USRDS, 2010). Renal transplantation patients at risk for graft thrombosis are those with previous vascular access thrombosis, previous venous thrombosis, antiphospholipid antibodies, and previous large vein renal transplant thrombosis (DeLoughery, 2004). A hypercoagulable state can occur in the nephrotic syndrome with urinary loss of the natural anticoagulants antithrombin III, protein C, and protein S. Hyperhomocysteinemia is common in ESRD, and it has been associated with thrombophilia (Levey et al, 1998). Antiphospholipid antibodies are found in 30% to 50% of patients with systemic lupus erythematosus, a systemic cause of ESRD. Patients can be evaluated with the activated protein C resistance ratio (factor V Leiden mutation), protein C activity, protein S activity, antithrombin III activity, homocysteine level, prothrombin gene mutation, and antiphospholipid antibodies (DeLoughery, 2004; Irish, 2004). It is reasonable to screen for thrombophilia when a patient is admitted for renal transplantation, administer low-dose heparin intraoperatively to all recipients, and continue low-molecular-weight heparin therapy until the results of the thrombophilia panel are known. On the basis of the thrombophilia panel results, a decision can be made about intermediate or long-term anticoagulation. The purposes of the urologic evaluation are to determine the suitability of the urinary bladder or its substitute for urinary tract reconstruction and to determine the necessity for removal of the native kidneys before or at the time of renal transplantation. The urologic evaluation includes a history for urologic disease and operations on the urinary tract; a physical examination including the location of scars, abdominal catheters, and stomas that may interfere with transplantation; urinalysis; urine or bladder wash culturing; and ultrasonography of the abdomen and pelvis to include a postvoid bladder image, the kidneys, and the gallbladder. Further study of the urinary tract is indicated for a history of urologic abnormalities, nonglomerular hematuria, single-organism bacteriuria, calculi, hydronephrosis, autosomal dominant polycystic kidney disease, significant bladder residual urine, or inconclusive preliminary imaging studies (Table 44–3). The anuric patient will need to be queried about bladder function that was present before urine production ceased. In chronic peritoneal dialysis patients, ultrasound examination of the bladder may overestimate residual urine. Table 44–3 Recommendations for Additional Urologic Studies in Renal Transplant Candidates Modified from Barry JM. Current status of renal transplantation: patient evaluations and outcomes. Urol Clin North Am 2001;28:677. An ESRD patient with upper urinary tract diversion may have a bladder that is acceptable for transplantation, especially if the original reason for diversion was vesicoureteral reflux. A defunctionalized bladder usually regains normal volume within weeks of transplantation. It is reasonable that transplantation candidates with small, contracted bladders who have had multiple lower urinary tract operations undergo bladder biopsy. If fibrosis is extensive on histologic examination and the bladder cannot be coaxed into becoming a low-pressure reservoir with bladder cycling, autoaugmentation or augmentation cystoplasty can be done before renal transplantation or afterward if satisfactory bladder function does not return within a few months (Barry, 2004). Functionalized augmentation is preferable to dry augmentation because it permits continence and bladder compliance to be documented before transplantation (Gonzales, 1997). A urothelium-lined augmentation is best because mucus does not have to be rinsed from the bladder on a regular basis. Advantages of gastrocystoplasty over enterocystoplasty are the lack of metabolic acidosis and significant mucus with the former. Disadvantages of gastrocystoplasty are the frequency-urgency syndrome due to acid from gastric mucosa and recalcitrant metabolic alkalosis. Patients with gastrocystoplasty who become anuric are at risk for bladder ulceration because acid produced by the gastric segment is not diluted with urine and washed out with voiding. Patients with augmented bladders usually require clean intermittent catheterization after transplantation, and the patient should be trained in this technique well in advance of the transplantation procedure. Clean intermittent self-catheterization has been used successfully in transplant recipients for more than 2 decades for patients with neuropathic bladders or transient bladder outlet obstruction (Schneidman et al, 1984). Cyclophosphamide is commonly used to treat immune complex glomerulonephritis, and a bladder wash for cytologic examination is recommended for patients with prior cyclophosphamide therapy because of its reported association with transitional cell carcinoma (Radis et al, 1995). The generally accepted recommendations for pretransplantation nephrectomy are outlined in Table 44–4. Renal calculi can be managed by minimally invasive techniques, and severe proteinuria and large polycystic kidneys without infection or solid tumor can be managed by renal infarction (Ubara et al, 2002). The incidence of renal cell carcinoma is more common in patients with acquired renal cystic disease and in transplant recipients than in the general population, and nephrectomy is indicated for solid renal tumors (MacDougall et al, 1990). If a polycystic kidney extends below the iliac crest, it is best removed to allow room for a kidney transplant. Pretransplantation nephrectomy is usually performed 6 weeks before transplantation or active wait-listing for deceased donor renal transplantation to allow wound healing and the detection and treatment of surgical complications. It is common to do kidney removal at the time of renal transplantation in children. It is common to do preliminary nephrectomies in adults, especially when the patient is to be listed for deceased donor renal transplantation, recurrent pyelonephritis has been noted, or when difficult nephrectomy with possible adjacent organ injury is anticipated. Bilateral nephrectomies for autosomal dominant polycystic kidney disease (ADPKD) at the time of renal transplantation have been reported (Glassman et al, 2000). The authors reserve this option for ADPKD patients who are undergoing living donor renal transplantation (Wagner et al, 2007). The use of synthetic erythropoietin has reduced the effects of anemia associated with nephrectomy while the patient awaits transplantation. Although laparoscopic or retroperitoneoscopic nephrectomies are becoming more commonly performed in these patients, bilateral vertical lumbotomies are quickly performed and well tolerated by patients with small kidneys undergoing nephrectomy (Freed, 1976). An open-flank approach is often used when a kidney is too large to be removed by a vertical lumbotomy or a laparoscopic technique. The open-abdominal approach is used when the kidneys are too large to remove with a minimally invasive technique or when additional procedures such as total ureterectomy, intestinal conduit removal, augmentation cystoplasty, the creation of a continent intestinal pouch, or the kidney transplantation are to be done at the same time. Table 44–4 Recommendations for Pretransplant Nephrectomy On the basis of the preoperative evaluation, one must be able to assure the living donor of nearly normal renal function after unilateral nephrectomy. On evaluation, if one of the potential donor’s kidneys is better than the other, the better kidney is left with the donor (Murray and Harrison, 1963). It is preferable to use the right kidney from women who may become pregnant because hydronephrosis of pregnancy and pyelonephritis of pregnancy occur predominantly in that kidney (Stevens, 1933; Monga, 1998). Although renal donation poses no great harm to pregnancy (Ibrahim et al, 2009a), it is recommended that pregnancy be delayed at least a year after nephrectomy (Nevis and Garg, 2009). An algorithm for the evaluation of a living renal donor is presented in Figure 44–2. Circumstances may change the order in which data are obtained. A living renal donor is considered to be unsuitable when one of the following is present: significant mental dysfunction, significant renal disease, high risk of perioperative mortality or morbidity, or significant transmissible disease. ABO incompatibility and a positive crossmatch between donor lymphocytes and recipient serum were usually considered to be contraindications to directed living renal donation, but immunosuppression protocols (Takahashi, 2007; Warren et al, 2004) and paired donation and altruistic chain transplant programs (Rees et al, 2009) are evolving to deal with those issues. Serologic testing is performed for HIV, human T-lymphoproliferative virus type 1 (HTLV 1), hepatitis, cytomegalovirus (CMV), and syphilis. Some programs also screen for Epstein-Barr virus, especially when the recipient is a child. Diabetes mellitus is excluded in the donor by determining that the casual or random plasma glucose level is less than 200 mg/dL, the fasting plasma glucose level is less than 125 mg/dL, or after a 75-g glucose load, the 2-hour plasma glucose level is less than 200 mg/dL (Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 1997). Abdominal ultrasonography can be performed to exclude donors with significant renal abnormalities and to detect incidental intra-abdominal abnormalities. Three-dimensional CT angiography without and with intravenous (IV) contrast material followed by a flat plate of the abdomen has been widely accepted because it satisfactorily excludes stone disease, demonstrates renal and vascular anatomy, and defines the urinary collecting system, all with minimal donor morbidity and at reasonable expense. Hyperfiltration injury has not been a significant problem for living renal donors. Endogenous creatinine clearance rapidly approaches 70% to 80% of the preoperative level, and this has been shown to be sustained for more than 10 years (Najarian et al, 1992; Ibrahim et al, 2009b). The development of late hypertension is nearly the same as for the general population, and the development of proteinuria is negligible (Steckler et al, 1990; Kasiske et al, 1995). Older donor age and increased body mass index (BMI) have been associated with the development of hypertension and GFR under 60 mL/min/1.73 m2 (Ibrahim et al, 2009b), and the authors disqualify potential living renal donors with BMIs over 33. The mortality of kidney donation has been estimated to be 0.02% (Matas et al, 2003), the risk of a potentially life-threatening or permanently debilitating complication has been estimated to be 0.23%, and there are isolated reports of ESRD developing in renal donors (Rosenblatt et al, 2008). The short- and long-term risks of living donor nephrectomy are generally considered to be low enough, and the probability of successful graft outcome high enough, to make the risks acceptable for fully informed donors. Live donor nephrectomy can be performed either as an open or laparoscopically assisted procedure. Open live donor nephrectomy (ODN) can be performed through a flank incision with or without rib resection or by an anterior intraperitoneal or extraperitoneal incision. Laparoscopic donor nephrectomy (LDN) has become the preferred technique for live donor nephrectomy at most transplant centers in the United States (Ratner et al, 1999; Jacobs et al, 2004), and a retroperitoneoscopic approach for either kidney has been described (Yang et al, 2001). Regardless of the technique of living donor nephrectomy, diuresis is promoted by IV fluids, mannitol infusion, and loop of Henle diuretics. Overnight hydration of the living donor is unnecessary. It is common to prepare the LDN candidate with a clear liquid diet and a laxative the day before surgery. General anesthesia is used. Epidural analgesia for pain management is optional. Nitrous oxide inhalation is avoided to prevent bowel distension. IV fluids and mannitol are used to prevent oliguria associated with pneumoperitoneum. The patient is placed in a modified right lateral decubitus position with the left side elevated approximately 45 degrees with a large gel roll. The table is partially flexed; it is unnecessary to raise the kidney rest. The patient is secured to the table with 2-inch cloth tape crossing just below the anterior superior iliac spines. This will permit rotation of the table during the procedure. Following the skin preparation and draping, the hand assist device is placed in a 7-cm periumbilical incision. The abdomen is insufflated to 15 mm Hg and inspected with a 10-mm, 30-degree telescope placed through a trocar in the hand assist device. Two 12-mm trocars are inserted under direct vision in the left lower quadrant and the subcostal regions, respectively (Fig. 44–3). These two trocars are generally all that are necessary. Occasionally, a third trocar (5 mm) is placed in the midaxillary line for lateral retraction. The advantages of LDN when compared with historical cases of ODN have been decreased analgesic requirement, decreased length of hospital stay, and an earlier return to work. The disadvantages have been reported to be increased operating time, donor surgeon preference for the left kidney, an increased need for special equipment, a longer warm ischemia time, and delayed graft function (Jacobs et al, 2004; Barry, 2005). Although initial renal transplant function may be somewhat slower to recover after LDN, single-center, longer-term results are not significantly different from those of ODN (Goel et al, 2004; Tooher et al, 2004). Earlier registry data indicated that 3-year kidney transplant survivals and projected half-lives of kidney transplants significantly favored the ODN flank approach over the LDN approach (Cecka, 2003). A difference in favor of ODN has been reported with the transplantation of adult kidneys into children (Troppman et al, 2005). As LDN teams become more experienced with the technique, these differences are expected to become insignificant, and emerging randomized trials of ODN versus LDN have reported equivalent renal transplant outcomes (Simforoosh et al, 2005). Although LDN for right kidneys was initially associated with poorer results due to thrombosis of the shorter right renal vein, further experience with this technique yielded results equivalent to those reported for left LDN (Posselt et al, 2004). A single-port laparoscopic donor nephrectomy technique has been recently described (Gill et al, 2008). The criteria for an ideal deceased kidney donor are normal renal function, no hypertension requiring treatment, no diabetes mellitus, no malignancy other than a primary brain tumor or treated superficial skin cancer, no generalized viral or bacterial infection, acceptable urinalysis, age between 6 and 50 years, and negative assays for syphilis, hepatitis, HIV, and human T-lymphoproliferative virus. Viral nucleic acid testing (NAT) is becoming more widely applied. It reduces infectious risk by shortening the period between infection and detectability (Kucirka et al, 2009). In an effort to expand the donor pool, the concepts of ECDs and DCDs have been adopted. ECDs are defined as donors older than 60 years old or donors 51 to 59 years old with any two of the following risk factors: cerebrovascular death, hypertension, and serum creatinine level greater than 1.5 mg/dL (Cecka, 2004). ECD kidney transplant survival rates, those from donors after cardiac death, and those from very young donors are significantly inferior to those from ideal deceased donor kidney transplants. The poorer graft survivals for kidneys from the very young are due to small anatomic parts and the risk of technical problems. There are, however, reports of successful kidney transplants from small deceased donors (Hudnall et al, 1989; Bretan et al, 1997). The transplantation of both kidneys from a deceased donor with abnormal renal function or abnormal biopsy findings has also been used with success as an option to expand the donor pool, but the long-term function of such kidneys is inferior (Light, 1999; Wolters et al, 2005). Table 44–5 shows biopsy guidelines that some have found useful for the selection of transplantable kidneys from expanded-criteria deceased kidney donors. The initial goals of resuscitation of the brain-dead deceased donor are generally systolic blood pressure of 90 mm Hg or mean arterial pressure of 60 mm Hg and urinary output exceeding 0.5 mL/kg/hr. Monitoring of central venous pressure, capillary wedge pressure, or pulmonary artery pressure is helpful for managing fluid administration (Boyd et al, 1991; Soifer and Gelb, 1989; Wood et al, 2004). Serum electrolyte levels are checked every 2 to 4 hours. If the resuscitation goals cannot be met by fluid challenge and the central venous pressure is greater than 15 cm H2O, dopamine or dobutamine at less than 10 µg/kg/min can be infused without causing renal vasospasm. Attempts are made to maintain a hematocrit greater than 24%, platelets greater than 10,000/microliter, international normalized ration (INR) less than 1.8, fibrinogen greater than 100 mg/dL, temperature 35° C to 38° C, and blood glucose 80 to 110 mg/dL. Blood is cultured if the donor has been hospitalized for more than 72 hours. Most renal donors are now multiple organ donors, and the classic abdominal midline and cruciate incisions have been largely abandoned in favor of the total midline approach with median sternotomy, even when kidneys alone are retrieved. The principles of deceased donor organ retrieval are adequate exposure, control of the vessels above and below the organs to be removed, initiation of preservation in situ, the removal of the organs, separation of the organs, completion of preservation, removal of histocompatibility specimens, removal of iliac vessels for vascular reconstruction of pancreas and liver grafts, and organ packaging. The technical aspects of an en bloc method are illustrated in Figure 44–4 (Nakazato et al, 1992; Barry, 1996). Some retrieval teams prefer to do much more dissection in situ before organ removal. When that occurs, the kidneys are the last transplantable organs to be removed. To avoid renal warming in this situation, it is important to maintain surface cooling of the kidneys by surrounding them with ice slush during liver and pancreas recovery. (A and B, From Barry JM. Cadaver donor nephrectomy. In: Novick AC, Streem SB, Pontes JL, editors. Stewart’s operative urology. Baltimore: Williams & Wilkins; 1989. p. 294–300; C-G, from Barry JM. Donor nephrectomy. In: Marshall FF, editor. Textbook of operative urology. Philadelphia: WB Saunders; 1996. p. 235–47.)
End-Stage Renal Disease
Incidence and Prevalence
Treatment Options
Results of Treatment
History of Human Renal Transplantation
Selection and Preparation of Kidney Transplant Recipients
Preliminary Screening
Kidney Disease Recurrence
Infection
Active Malignancy
High Probability of Perioperative Morbidity or Mortality
Noncompliance
Unsuitable Conditions for Technical Success
STUDIES
INDICATIONS
Voiding cystourethrogram ± urodynamics
Voiding dysfunction, history of pyelonephritis or reflux, inconclusive ultrasonography
Cystoscopy
Suspected lower urinary tract cancer or planned invasive prostate therapy
Retrograde pyelography
Planned orthotopic renal transplantation or inconclusive ultrasonography
Renal CT scan
Inconclusive ultrasonography for stone or mass, autosomal dominant polycystic kidney disease to accurately size kidneys
Urine or bladder wash cytology
Prior cyclophosphamide therapy or significant irritative voiding symptoms
Bladder biopsy
Suspected bladder fibrosis or cancer
Retrograde loopogram
Intestinal conduit
Retrograde pouchogram
Intestinal or gastric reservoir
Donor Selection, Preparation, and Surgery
Living Donor
Technique of Laparoscopic Live Donor Nephrectomy
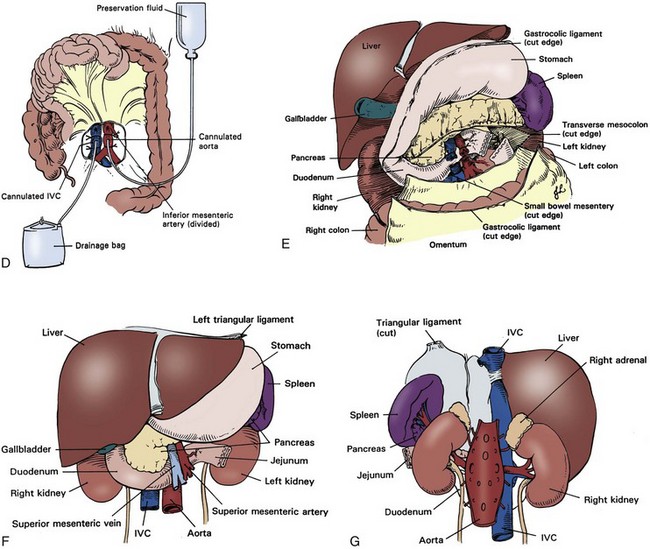
Deceased Donor
Abdominal Key
Fastest Abdominal Insight Engine