Fig. 15.1
(a) Confluent epithelial cells were strongly connected to each other and attached to the temperature-responsive surfaces of the culture dishes at 36 °C. (b) Cell sheets were harvested by decreasing the temperature to 20 °C, without use of any enzymes
Treatment for the Prevention of Esophageal Strictures: Autologous Oral Mucosal Epithelial Cell Sheets
We developed a treatment adapting regenerative medicine using tissue-engineered epithelial cell sheets [15–17]. Epithelial cells isolated from the patient’s own oral mucosa (Fig. 15.2a) were seeded onto temperature-responsive culture inserts (Fig. 15.2b) and cultured for 16 days at 36 °C (Fig. 15.2c, d). The autologous cell sheets (Figs. 15.2e and 15.3) were then transplanted with endoscopic forceps onto the bed of the esophageal ulcer after ESD (Figs. 15.2f and 15.4). Nine cases involving oral mucosal epithelial cell sheet transplantation have been reported [17]. The results indicate that cell sheet transplantation effectively prevents post-esophageal ESD strictures (Fig. 15.5).
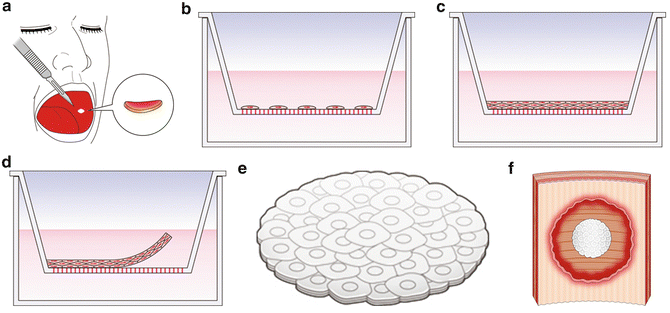

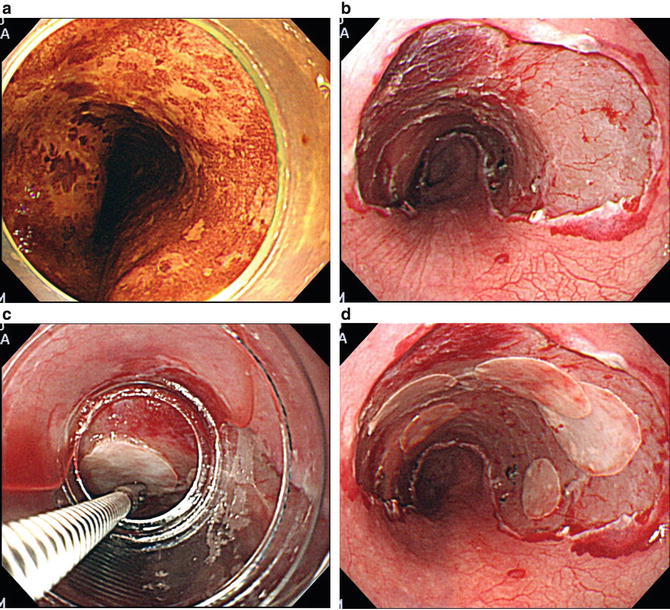
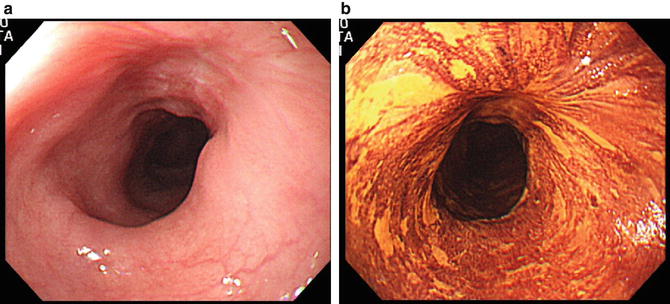

Fig. 15.2
(a) The patients’ own oral mucosal tissues were used to obtain cells for culture. (b) Isolated cells were seeded onto temperature-responsive culture inserts. (c) Epithelial cells on temperature-responsive culture inserts were cultured by confluent growth for 16 days. (d) Epithelial cell sheets were harvested by reducing the temperature to 20 °C. (e) Harvested autologous oral mucosal epithelial cell sheet. (f) Cell sheets were endoscopically transplanted to the affected area immediately after esophageal ESD

Fig. 15.3
Autologous oral mucosal epithelial cell sheet

Fig. 15.4
Endoscopic view of a typical case where esophageal strictures were prevented using cell sheets. (a) Three quarters of the circumferential tumor is shown as an unstaining area with iodine dye chromoendoscopy. (b) An artificial ulceration immediately after ESD. (c) Support membranes attached to the epithelial cell sheet were grasped with endoscopic forceps and transplanted to the affected area by endoscopy. (d) The affected area immediately after endoscopic transplantation of cell sheets

Fig. 15.5
(a) Three weeks after cell sheet transplantation; visualized under white light. (b) Three weeks after transplantation; visualized as well stained area with iodine dye chromoendoscopy
Culture of Autologous Oral Mucosal Epithelial Cell Sheets
After each patient’s oral cavity was sterilized, specimens of oral mucosal tissue were surgically taken from the buccal mucosa (Fig. 15.2a). Two types of specimens were used for the cell culture: multiple 6 mm diameter circular tissue samples and spindle-shaped tissue samples [18]. Oral mucosal epithelial cells were collected by removing all epithelial layers with dispase I (1,000 PU per milliliter, Godo Shusei, Tokyo, Japan) at 37 °C for 2 h. Next, the cells were placed in trypsin and ethylenediaminetetraacetic acid (EDTA) for 20 min to form single-cell suspensions. Temperature-responsive cell culture inserts (UpCell Insert, CellSeed, Tokyo, Japan) (23.4 mm in diameter) were prepared as previously described [19, 20]. Isolated cells were seeded onto these temperature-responsive culture inserts (Fig. 15.2a) and cultured with keratinocyte culture medium (KCM) (Table 15.1) by confluent growth for 16 days (Fig. 15.2c, d).
Table 15.1
Keratinocyte culture medium (KCM) for harvest of oral mucosal epithelial cell sheets
Culture medium | Dulbecco’s modified Eagle’s medium (DMEM); Sigma, St. Louis, MO | (3:1 DMEM:Ham’s F-12) |
|---|---|---|
Ham’s F-12; Sigma, St. Louis, MO | ||
Media supplements | 2 nmol/L triiodothyronine; Wako Pure Chemicals, Osaka, Japan | |
5 μg/mL insulin; Eli Lilly, Indianapolis, IN | ||
10 ng/mL epidermal growth factor (EGF); Higeta Shoyu, Chiba, Japan | ||
0.4 μg/mL hydrocortisone; Kowa Pharmaceutical, Tokyo, Japan | ||
1 nM cholera toxin; List Biological Laboratories, Campbell, CA | ||
0.25 μg/mL amphotericin B; Bristol-Myers Squibb, New York, NY | ||
40 μg/mL gentamicin; Schering-Plough, Kenilworth, NJ | ||
5 % autologous human serum | ||
Preparation for Transplantation
Two incubators (36 and 20 °C) were used in an endoscopic procedure room to harvest the cell sheets. The patients’ cell sheets were transferred from the cell processing center to the 36 °C incubator. The cell culture team staff carried those sheets to another incubator (20 °C) and harvested the cell sheets (Figs. 15.2e and 15.3) as instructed by the ESD operator. A sheet of polyvinylidene difluoride (PVDF) (Immobilon-P, Durapore; Millipore, Billerica, MA) was used as a support membrane.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







