Fig 25.1
Voiding cystourethrogram revealed right vesicoureteral reflux to blunted calices (not shown). Images provided by Dr. Akira Kawashima, Department of Radiology, Mayo Clinic, Rochester, MN
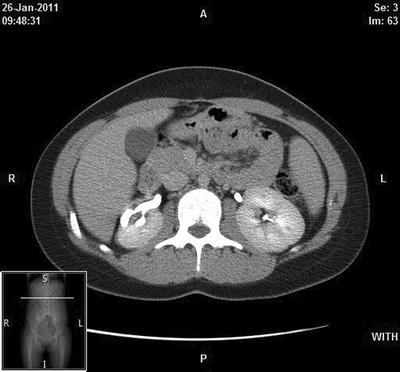
Fig 25.2
Delayed enhanced CT scan obtained at the level of the right mid-kidney demonstrates a dilated calyx with associated cortical scanning laterally, characteristic of chronic atrophic pyelonephritis. Images provided by Dr. Akira Kawashima, Department of Radiology, Mayo Clinic, Rochester, MN
Historically, reflux nephropathy was initially understood to be renal parenchymal lesions found in the kidneys of patients who experienced a febrile urinary tract infection or pyelonephritis in the face of VUR [7, 8]. A classic cascade of events due to bacterial infection of the kidneys was well described by Roberts [9]. Schematically the process of renal scarring starts with the colonization of the urinary tract by a bacterial organism such as Escherichia coli which is capable of adhering to the urothelium. Colonization occurs because of many factors including ineffective bladder emptying, decreased immune response, and urologic anomalies such as vesicoureteral reflux. With ascent of urine up into the kidney, the bacteria can enter the renal parenchyma and cause pyelonephritis as demonstrated by Ransley and Risdon who referred to the event as the “big bang” effect [10, 11]. Development of renal scarring is dependent on the inflammatory response in the renal medulla initiated by invasion of the renal tissue by bacteria. A local inflammatory response will develop and may result, ultimately in local renal tissue damage. Roberts has postulated that bacteria adhering to the renotubular cells may elicit an immune response which causes release of superoxide which will, in turn, damage both bacteria and tubular cells. The damage subsequently causes an interstitial inflammatory response which leads to deposition of collagen and destruction of the normal tubular arrangement.
A schematic representation of cascade events causing renal damage in the face of bacterial invasion is described on Fig. 25.3. The final outcome is seen as areas of the kidneys which are replaced by areas of scar tissue and damaged nephrons [12, 13]. The pathological features of chronic pyelonephritis can be identified microscopically as areas of fibrosis and cortical thinning overlying dilated and distorted calyces (Figs. 25.4 and 25.5). The “scarred” kidney will usually be seen to be smaller than normal with variability in the areas of scarring. The scarred area shows dilated and atrophic tubules with a preservation of the large blood vessels. Normal parenchyma is usually seen adjacent to areas of scar. Periglomerular fibrosis and varying degrees of glomerular sclerosis can also be observed [13]. The changes are not exclusive of the area of scarring but can also be seen in normal areas of the kidneys which may indicate that the autoimmune process of the renal damage may be at work diffusely in patients who have had a pyelonephritis. Anomalies may also be noted and include periarterial fibrosis and changes consistent with medullary fibrosis.
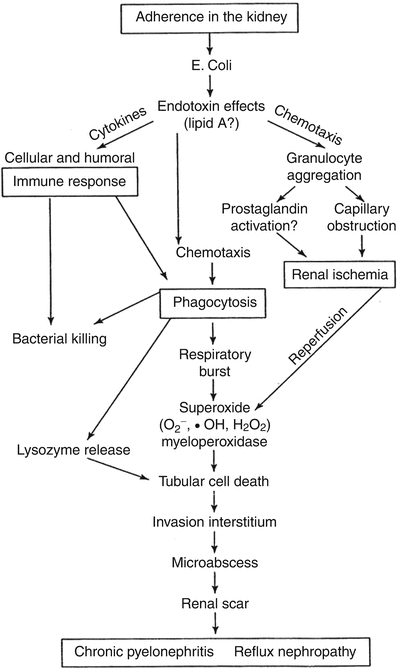
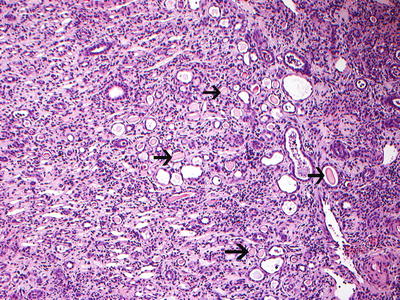
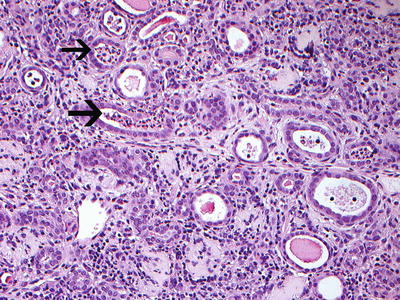

Fig. 25.3
Schematic representation of the pathologic events in the formation of pyelonephritic scar. From: Roberts JA. Vesicoureteral reflux and pyelonephritis in the monkey: a review. J Urol 1992;148(5 Pt 2):1721–5. With permission

Fig 25.4
Reflux nephropathy showing features of chronic pyelonephritis. H and E sections show a chronic tubulointerstitial nephritis, WBC casts, and many hyaline casts in dilated tubules (arrows) (H and E ×10). Courtesy Dr. Sanjeev Sethi, Department of Radiology, Mayo Clinic, Rochester, MN

Fig 25.5
Chronic pyelonephritis with WBC casts (arrows). Note ruptured tubule with WBC cast (H and E ×20). Courtesy Dr. Sanjeev Sethi, Department of Radiology, Mayo Clinic, Rochester, MN
Recently, the etiologic implication of infection as the sole cause of renal damage in reflux nephropathy has been challenged because some patients with VUR have no documentation of infection present with evidence of renal damage as documented by either ultrasound or (more specifically) by DMSA scan. These renal lesions may, in fact, be different than those associated with urinary tract infection and are referred to renal dysplasia [14]. Renal dysplasia is a pathological diagnosis in which evidence of primitive renal tissue is found in association with significant medullary fibrosis and areas of abnormal tissue such as cartilage. Renal dysplasia is found commonly in other conditions such as posterior urethral valves, upper urinary tract obstruction, and duplicated collecting systems with upper pole pathology and can be classified as either cystic or solid [15]. Mackie and Stephens theorized that renal dysplasia may actually be the result of altered kidney development associated with abnormalities in the embryology of the mesonephric duct and metanephric blastema [16]. It has become clear over the last few years that a number of signaling molecules and transcription factors play an important role in the developmental process of the ureteral bud and of the kidney. Abnormalities in ureteral bud development and improper induction of the metanephric blastema may occur when expression of these molecules is altered leading to a field defect resulting in both kidney and the urinary tract abnormalities. Several animal studies using mouse models point towards a complex system of receptors and transcription factors which influence the growth and elongation of the distal ureter (GDNF/RET-signaling pathway) [17]. The theory of Mackie and Stephens regarding abnormal renal development in the face of abnormal ureteral bud insertion is being confirmed on a molecular basis in these animal models [18]. Current understanding is that the mesenchyme adjacent to abnormally positioned ureteral bud may not be competent to respond to inductive signals and this leads to abnormal renal development more specifically renal dysplasia.
Obstruction to the normal flow of urine from the kidneys has also been postulated as a possible etiologic factor for renal dysplasia [19].
It is therefore felt that reflux nephropathy associated with VUR may be a spectrum of disease and close attention should be paid to the etiologic factors of the renal abnormalities observed. In general, renal dysplasia is seen in patients diagnosed prenatally or early in life who have not had any evidence of urinary tract infection [20]. The features of renal dysplasia on DMSA scan usually are that of a diffuse, reduced uptake of the radionuclide; whereas reflux nephropathy associated with recurrent urinary tract infection is seen focally, the defects of DMSA scan appear to be localized (segmental) in the upper and lower poles and associated with relatively high grade reflux [21, 22]. As will be discussed later, these distinctions are important in managing children with reflux nephropathy.
Pathophysiology of Vesicoureteral Reflux
Vesicoureteral reflux is, in all likelihood, the result of maldevelopment or immaturity of the ureterovesical junction (UVJ). Histologically, the distal ureter is seen to enter the outer muscle layer of the bladder and then course underneath the mucosa of the bladder. This arrangement has been postulated to act as a flap-valve mechanism whereabouts the submucosal roof of the submucosal portion of the ureter will be compressed as the bladder fills. Urine will still be allowed to flow down the ureter as the peristaltic activity of the ureteral muscle will propel the urine away from the kidney. The length of intramural tunnel is felt to be important. If the length of ureter underneath the mucosa of the bladder, or if the diameter of the distal ureter is large, the flap-valve mechanism will be ineffective and ascent of urine up the ureter will occur. Other factors may, in fact, be involved such as the anchoring of the ureter to the muscle of the bladder. Paquin, in 1959, showed that the length of intramural tunnel is essential to preventing the reflux of urine. He postulated that a normal, non-refluxing ureter had a tunnel length to ureteral diameter ratio of 5 to 1, whereas in refluxing ureters that ratio was much smaller [23]. The appearance of the ureteral orifice may not be important but its location within the bladder is certainly crucial as more laterally displaced ureteral orifices will indeed be associated with reflux. The histologic events associated with the development of the ureterovesical junction have been well described by F. Douglas Stephens and may explain the development of a faulty ureterovesical valve [24].
Reflux of urine from the bladder back up into the ureter and renal collecting system has been recognized since the time of Galen [25]. VUR became identified as an etiologic factor for pyelonephritis from the classic studies by Hutch who, in 1952, studied a group of paraplegic patients diagnosed with neurogenic dysfunction of the bladder and vesicoureteral reflux. Reflux of infected urine into the upper urinary tract was hypothesized to be the cause of chronic pyelonephritis and subsequent renal damage [26]. Subsequently Hodson in 1959 observed that reflux seemed to be more common in children with urinary tract infection and that there was a correlation between reflux and chronic pyelonephritis as documented by VCUG (voiding cystourethrogram) and IVU (intravenous urogram) [27]. The increasing use of imaging studies of the urinary tract such as the VCUG, led to the recognition that reflux is associated with upper urinary tract infections and renal parenchymal lesions. The presence of intrarenal reflux (reflux of contrast into the medulla of the kidney) which can be occasionally demonstrated on VCUG has also been associated with renal scarring [28].
When the association between vesicoureteral reflux and urinary tract infection became more established, additional information became available and a relationship between renal abnormalities and reflux was also observed. In the early 1970s, Rolleston reported that severe reflux in infants seemed to have a higher likelihood of associated renal damage [8]. This led to the belief that renal damage associated with vesicoureteral reflux may be acquired and was caused by recurrent, ascending urinary tract infection. However, this notion was disputed by Stecker and associates who reported the presence of renal parenchymal lesions which were found in a small series of patients with reflux but who had never had any evidence of a urinary tract infection [29].
Presentation of Vesicoureteral Reflux
Primary vesicoureteral reflux can be classified by its mode of presentation. Until the advent of prenatal screening by ultrasound, vesicoureteral reflux was usually identified during a work-up of a febrile urinary tract infection. However, over the last 20 years it has become apparent that reflux can be detected prior to the advent of a urinary tract infection. Prenatal ultrasound has revealed a presence of variable degrees of hydronephrosis in a significant number of fetuses. Postnatal evaluation with VCUGs has revealed the presence of vesicoureteral reflux in a significant number of cases (approx. 20 %) [30]. Although vesicoureteral reflux cannot be diagnosed in utero, its presence can be inferred from findings on conventional ultrasound during prenatal screening. Radiologic findings include variation in the degree of dilatation of the upper urinary tract during prolonged observation of the fetus with increasing size of the upper urinary tract during emptying of the bladder [31]. Such findings should lead to postnatal evaluation with a VCUG [32].
Prenatal hydronephrosis is mostly reported in males, can be variable in its degree and grade, and is not associated with urinary tract infection. Renal abnormalities have also been observed in patients found to have VUR diagnosed in the perinatal period. The extent of the renal abnormalities can vary with up to 10 % of patients showing a poorly or nonfunctioning kidney on the side of the reflux [2].
The classic presentation of vesicoureteral reflux after birth is in the setting of a febrile urinary tract infection (UTI) occurring in an infant or an older child. The features of this type of vesicoureteral reflux are that it usually affects females, is usually diagnosed later in life (especially during toilet training time), and is usually associated with lower grades of vesicoureteral reflux [33]. In addition, 25–50 % of patients who present with acute pyelonephritis are found to have VUR. It should be emphasized, however, that the relationship of VUR and UTI is not cause and effect. One key concept is that, in the face of VUR, bacteria that have entered the bladder have easy access to the upper urinary tract [34]. Most children who have lower grades of VUR and few UTIs have, in general, as they grow older, a benign outcome [35, 36]. Nevertheless, a concern exists in that renal lesions can be observed in up to 13.5 % of patients who have had recurrent urinary tract infections involving the upper urinary tract or pyelonephritis [37]. But the causal relationship between UTIs and renal scarring is difficult to define despite a fairly large body of literature based on mostly retrospective studies [38, 39].
Evidence suggests that several factors may contribute to renal damage and that a genetic predisposition may exist [40, 41]. The age at which a child with VUR has an episode of pyelonephritis and the number of episodes of infection appear to be related to the severity of renal damage. Pylkkanen et al. reported that infants younger than 1 year of age carry the highest risk of developing renal damage and the highest incidence of congenital urinary tract anomalies, whereas after puberty new renal damage does not appear to occur [41]. In addition, the occurrence of renal lesions is directly related to the frequency of the episodes of upper urinary tract infection; the more infections, the more renal damage is seen [42]. Genetic predisposition for renal damage in association with VUR has also been suggested [43, 44]. Association with ACE gene polymorphism with urinary tract infections and renal lesions in young children has been suggested [45]. Clearly the molecular aspects and the genetics of renal maldevelopment and injury seen in association with VUR will require further studies.
Approximately 30 % of siblings of patients with reflux will also be noted to have reflux [46, 47]. This category of patients usually does not have a history of urinary tract infection and, overall, seems to have a fairly good prognosis with few patients exhibiting any renal lesions [48].
The prevalence of vesicoureteral reflux has been hard to estimate as most patients present either prenatally, with a urinary tract infection or on family screening. A number of patients are diagnosed after being screened for reflux when familial reflux is reported [49]. Obviously a large portion of the population has not been screened for reflux but the incidence of vesicoureteral reflux has been estimated to be 1–2 % of live births [50]. Reflux is not distributed equally amongst all races. It appears that children of West African ancestry have very low incidence of VUR [51–54].
Diagnostic Evaluation of Vesicoureteral Reflux
Diagnosis of reflux is achieved by demonstrating retrograde flow of urine up into the kidney. This is best carried out by either a voiding cystourethrogram or a radionuclide cystogram. Each of these modalities will be described separately. As discussed earlier prenatal diagnosis of vesicoureteral reflux cannot be made but can be inferred by features of a well carried out prenatal ultrasound. In the early 1990s, with improvement in ultrasound technology, several articles described what is now referred to as prenatally diagnosed reflux [31, 55–57]. Ultrasound diagnosis prenatally of the possibility of vesicoureteral reflux is usually inferred when hydronephrosis is noted. A recent meta-analysis on the postnatal outcome of antenatally diagnosed hydronephrosis indicates that the overall risk of vesicoureteral reflux in the population of fetuses diagnosed with antenatal hydronephrosis is approximately 8.6 % [58]. An important feature of vesicoureteral reflux associated with antenatal hydronephrosis is that the degree of prenatal hydronephrosis does not correlate with the presence or degree of vesicoureteral reflux. In fact, the rate of vesicoureteral reflux in patients screened postnatally with VCUG seems to be pretty much constant in the various grades (mild, moderate, severe hydronephrosis ranging from 4.4 % presence of vesicoureteral reflux to 14 % in the moderate degrees and 8.5 % in the severe degree of hydronephrosis). It is therefore felt that hydronephrosis is an indicator of urologic pathology but is not necessarily a predictor of vesicoureteral reflux when diagnosed prenatally. If there is a suspicion for vesicoureteral reflux then postnatal evaluation is warranted. The optimal timing of the postnatal follow-up remains debatable, but current recommendations are for an ultrasound 2–3 days after birth and a VCUG or RNC (radionuclide cystogram) within a month after birth. In general, a VCUG is also recommended in females who have not had a urinary tract infection and have a family history of vesicoureteral reflux or hydronephrosis diagnosed prenatally. In males, a VCUG is favored since it will provide better anatomic resolution and will help rule out any lower urinary tract abnormalities such as posterior urethral valves.
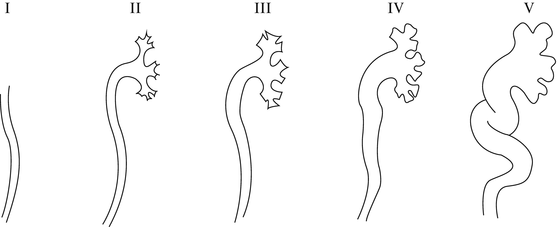
The voiding cystourethrogram is the principle method of assessing the lower urinary tract in children. The first images of the bladder and urethra were reported back in 1905, but it was not until the early 1930s that instillation of contrast material into the bladder was evaluated fluoroscopically during voiding [59]. VCUG is carried out by inserting a catheter in the bladder. The bladder is filled until the child voids. Sequential images of the voiding phase are obtained. Filling and emptying the bladder (cycling) has been shown to increase the yield for the diagnosis of vesicoureteral reflux [60]. VCUG will allow quantification of the amount of reflux and will outline both the bladder and the upper urinary tract anatomy as well as the anatomy of the urethra [61]. As noted the current accepted classification of the severity of reflux is based on VCUG findings (Fig. 25.5) [62]. Other pathological conditions of the bladder can be noted on VCUG such as bladder diverticula which are associated with reflux as well as posterior urethral valves, neurogenic bladder dysfunction, urethral strictures, and ureteroceles as well as reflux into one or both poles of a duplex kidney.
Bladder conditions associated with vesicoureteral reflux include:
Prune-Belly syndrome
Duplication of the upper urinary tract
Posterior urethral valves
Neurogenic bladder
Bladder diverticulum
Ureterocele
The VCUG is recognized to be a fairly invasive test, which may cause a significant amount of psychological stress to the child if not performed correctly by those skilled in the imaging of children. In addition, use of ionizing radiation near the growing child’s gonads from VCUG is concerning. Recent improvement in fluoroscopic techniques using digital techniques will significantly reduce the exposure to radiation [63].
The radionuclide cystogram (RNC) has also become widely accepted for the evaluation of vesicoureteral reflux. Its first practical application was described in the early 1960s and has become a popular way to evaluate for vesicoureteral reflux in children who are followed for reflux [3]. Urethral catheterization is required and a radionuclide solution is instilled into the bladder. The advantage of this technique is that the amount of radiation associated with the radionuclide is less than that with a VCUG [64, 65]. In addition, the nuclear cystogram may have a greater sensitivity as it provides continuous monitoring of the bladder as it fills and empties. However, no anatomic determination of the lower or upper urinary tract can be achieved using the nuclear cystogram. The classification of the reflux is also different as it can only show mild, moderate, or severe degrees (grades I, II, III). It is also sometimes hard to see grade I vesicoureteral reflux (international reflux classification) (Fig. 25.6) on a radionuclide cystogram because of the activity in the bladder. The limitations of the radionuclide cystogram have led some authors to favor the VCUG as the initial study to diagnose reflux. The radionuclide cystogram is then used for subsequent follow-up studies [66, 67]. Cycling the bladder will increase the sensitivities as described by Fettich and Kenda [68]. To avoid catheterization of the bladder, several alternate techniques have been attempted to diagnose vesicoureteral reflux but none have been shown to have the diagnostic accuracy of either the VCUG or RNC. For example, the indirect radionuclide cystogram (IRC) described by Merrick et al. has been associated with a high rate of false-negative and false-positive studies [69, 70].
In order to fully evaluate the urinary tract in the face of vesicoureteral reflux, current recommendations are to carry out an ultrasound study which will give anatomic details of the kidneys as well as indications as to the anatomy of the upper collecting system. The ultrasound will also help to evaluate the lower urinary tract and show whether or not the bladder empties properly. However, ultrasound is not a very helpful test in monitoring kidneys except to evaluate for their growth. A large study by Blane and colleagues showed that a significant number of kidneys in patients with vesicoureteral reflux were normal by ultrasound (74 %) without evidence of ureteral or renal pelvic dilatation [71]. It was, therefore, felt that conventional renal ultrasonography is not a helpful test to diagnose reflux but can serve as a screening test to look for anomalies of either the bladder or kidney and acquired conditions.
With regard to renal abnormalities associated with vesicoureteral reflux, recent studies have suggested that the dimercaptosuccinic acid (DMSA) renal scan may be the most important study in the evaluation of patients who present with a urinary tract infection and vesicoureteral reflux. DMSA scan had been shown to be a useful tool in assessing both for acute and permanent renal damages in children with urinary tract infection [6, 72]. Evaluating a patient after a febrile urinary tract infection and diagnosed with vesicoureteral reflux may, in fact, help identify those at risk for long-term sequelae of vesicoureteral reflux nephropathy. DMSA uses an agent that labels tubular cells tubular which enables good cortical imaging of the kidneys and can be used to evaluate children with both urinary tract infections (UTI) and vesicoureteral reflux. DMSA scans can demonstrate evidence of pyelonephritis in the acute setting as well as evidence of permanent renal lesions if performed several months after resolution of the infection. There are, however, no published guidelines for the use of the DMSA in children with reflux. Clearly from the urologic and nephrologic standpoints, the presence of renal lesions is an important piece of information. A recent study of 303 children under age 2 who were diagnosed with UTI and investigated with a DMSA scan as well as a VCUG (top down approach to reflux) within 3 months after UTI demonstrated that 50 % of the patients showed renal lesions on DMSA scan. Only 26 % of those patients, however, had vesicoureteral reflux. Of note is that the grade of vesicoureteral reflux correlated significantly with the presence of renal lesions [73].
DMSA scanning has been shown to be more accurate than intravenous urography (IVU) in evaluating for the presence of renal lesions subsequent to urinary tract infection in the face of reflux [4, 5]. Rushton and colleagues followed patients with acute pyelonephritis with serial DMSA scan at the time of the acute episode and then several months later. They observed that the areas identified as foci of acute pyelonephritis later appeared as areas of renal damage and therefore confirmed that acute pyelonephritis can lead to renal lesions [22]. Technical improvements have not led to ultrasonography replacing DMSA renal scanning in the ability to detect parenchymal lesions. In fact, small focal lesions less than 1 cm are not picked up by renal ultrasound [71]. Investigators have proposed that DMSA scan may be the study of choice in children with febrile urinary tract infection [74]. Mingin and colleagues suggested that abnormalities seen on DMSA scan correlated with the presence of grades III–V reflux in children with febrile urinary tract infection, and these children had a greater chance of having a breakthrough infection (60 %) than those without renal lesion [75]. DMSA may detect those patients who may be at risk for renal damage in the face of VUR and urinary tract infection. Therefore, it has been suggested that the DMSA scan be the first study in patients with a febrile UTI with evidence of upper urinary tract dilatation seen on ultrasound and that VCUG or RNC be carried out only in those patients with evidence of renal lesions on DMSA scan [73].
Magnetic resonance urography (MR urography) has been studied as potentially new diagnostic modality for renal and urinary tract evaluations. While MR urography may provide improved spatial and contrast resolution than DMSA scan, it remains an experimental tool as no definite criteria or categories of renal lesions have been published. It has been suggested, however, that MR urography may distinguish renal dysplasia from post-pyelonephritic renal lesions but further studies will be needed to validate this possibility [76]. It should be kept in mind that MR urography is more expensive and more time-consuming and requires anesthesia or sedation in the pediatric population.
The Natural History of Vesicoureteral Reflux
Vesicoureteral reflux is known to resolve spontaneously in a number of infants and children. Several studies, both prospective and retrospective, have tried to assess the rate of resolution [77–82]. Elder et al. following a thorough review of the literature, provided resolution curves that showed that reflux resolution was more likely to occur in younger children and that at 5 years, 92 % of patients with grade I and 81 % of patients with grade II reflux showed resolution of the reflux irrespective of the age at presentation and whether the reflux was unilateral or bilateral (Fig. 25.7) [38]. Unfortunately the data suffers from several problems including heterogenicity of definitions of the outcomes. Reflux grading has not been consistent in all studies and may not be comparable. Resolution rate has been looked at both in terms of ureters as well as patients. This makes the data hard to interpret. Several means of evaluating for reflux have also been reported upon and these include both voiding cystourethrograms as well as radionuclide cystograms. Also patient selection is not always consistent in some of these studies. Reflux has been shown in some series to be intermittent and reports of resolutions have then been modified when reappearance has been noted at a later study [50]. Finally, in all studies, a significant number of patients were noted to be lost to follow-up. More recently, a nomogram used to predict reflux resolution in primary vesicoureteral reflux showed that resolution was dependant on several factors which included age at presentation, gender, grade, laterality, mode of presentation, and ureteral anatomy [82]. Nevertheless, certain conclusions can be gleaned from the data. First of all, reflux resolution seems to occur more likely in younger children and children with lower grades of reflux (reflux grades I and II). Resolution of grade III reflux seemed to vary depending on laterality. If the reflux was unilateral and children were under age two, reflux disappeared in 70 % of cases. If the patient has bilateral grade III vesicoureteral reflux and was older than five, the resolution rate for reflux was much lower (12.5 %). For unilateral grade IV reflux, resolution rate was in the order of 58 % after 5 years, but if the patient had bilateral grade IV reflux, only 10 % demonstrated resolution (Table 25.1). Grade V reflux resolved in less than 5 % of patients [38, 82–85].
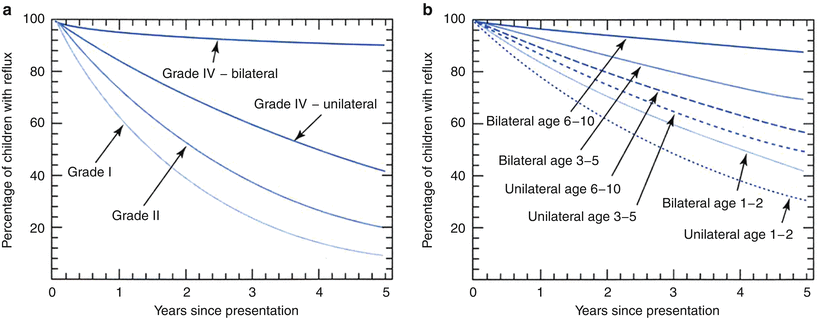

Fig. 25.7
Curves showing the likelihood of spontaneous reflux resolution in percentages of patients with VUR followed up to 5 years: (a) for VUR grades I, II, and IV and (b) for VUR grade III by patient age at presentation [38]
Series | No. of patients | Grade I (%) | Grade II (%) | Grade III (%) | Grade IV (%) |
|---|---|---|---|---|---|
Bellinger and Duckett [10] | 269a | 87 | 63 | 53 | 33 |
Goldraich and Goldraich [2] | 202 | 80(I/II) | 50(III/IV) | ||
Huang et al. [11] | 214 | 92 | 76 | 62 | 32 |
Greenfield et al. [12] | 601 | 69 | 56 | 49 | |
Smellie et al. [6] | 149 | 73b | 44b | ||
Schwab et al. [13] | 214 | 83 | 77 | 68 | 36 |
Estrada et al. 2007 (personal communication) | 86(I/II) | 72(III) | 54(IV/V) | ||
Resolution of reflux over time has been the proposed basis for medical management of vesicoureteral reflux. In addition to the age of the patient, the grade of reflux, and laterality, the status of bladder function has come to be considered as an important factor in reflux resolution. Koff, in 1992, recognized the relationship between abnormal voiding patterns and vesicoureteral reflux [86]. Subsequently, Snodgrass and Koff independently showed that children with vesicoureteral reflux and urinary tract infection showed symptoms of voiding dysfunction. Symptoms of voiding dysfunction include infrequent voiding, frequency-urgency, incomplete bladder emptying, and day and night incontinence [87, 88]. Patients with voiding dysfunction appear to have a higher rate of constipation and seem to be predisposed to urinary tract infection. Farhat and colleagues felt that a proper evaluation for children with vesicoureteral reflux and urinary tract infection should include investigation for voiding dysfunction. This group recommended the use of a validated dysfunctional scoring system using test questions geared at evaluating urinary and bowel habits to screen for patients who may have dysfunction elimination syndromes [89]. Using this questionnaire the Pediatric Urology Group from Toronto studied a large group of patients with vesicoureteral reflux. Children with high symptom score who were managed with behavior modification consisting in timed voiding with coordinated relaxation of the external sphincter and complete bladder emptying seemed to demonstrate a higher rate of reflux resolution which correlated to reduction in the symptom score. Children who did not have much reduction in the symptom score tended to demonstrate persistence of the reflux [90].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree