Rectal Cancer: Management of Locoregional Disease
Morton S. Kahlenberg
Dennis L. Rousseau Jr.
Jon Strasser
Adam Raben
Nicholas Petrelli
The evolution of the multidisciplinary management of rectal cancer has resulted in impressive local control rates, overall survival rates, and enhancements in quality of life. Surgical resection represents the focal point of the multidisciplinary management of patients with rectal cancer. Several anatomic factors make complete surgical extirpation of rectal cancer challenging. The absence of a serosal barrier and the rectum’s close proximity to vital pelvic structures facilitates early tumor extension into the perirectal tissues and contiguous organs. The location of the mesorectum within the confines of the pelvis complicates the adequate removal of all mesenteric nodes at risk for regional spread. The relationship between tumor location and sphincters and the priority to maintain continence through sphincter preservation and other quality-of-life issues adds to the challenge of the surgical management of rectal cancer. Treatment decisions have become more complex as a result of an increased array of surgical options and the excellent response of rectal cancer to chemotherapy and radiation. This chapter focuses on the locoregional management of rectal cancer. Radical resections (RADs) including sphincter preservation procedures and less radical procedures including transanal excision (TAE) and transanal endoscopic microsurgery (TEMS) are addressed. The roles of total mesorectal excision (TME), surgical margins, en bloc resection, and pelvic exenteration are discussed. The choice of surgical approach is dependent upon accurate pretreatment staging as well as the role that chemotherapy and radiation play in the neoadjuvant and adjuvant settings. This chapter will provide a brief overview of the approach to the pretreatment evaluation of the patient with rectal cancer, and the roles of neoadjuvant and adjuvant chemoradiation in rectal cancer management will be addressed.
Pretreatment Evaluation and Staging
The pretreatment evaluation and staging are essential in determining the surgical approach to rectal cancer and the order of the various treatment modalities (radiation, chemotherapy, and surgery). The determination of the extent of local involvement and the presence or absence of systemic disease is critical. Clinical determination of the local extent of disease (T and N stage) includes physical examination consisting of digital rectal examination. For experienced practitioners, digital rectal examination is fairly accurate in assessing the depth of rectal wall penetration but not the nodal status. Waizer et al. (1) reported that digital examinations performed by experienced practitioners accurately predicted the degree of rectal wall invasion 82.8% of the time. Computed tomography (CT) has been part of the standard preoperative staging for rectal cancer. It is very good for assessing the involvement of adjacent organs by locally advanced tumors but has limited accuracy in determining the T stage of tumors or the presence of nodal metastases. Several studies indicate that the accuracy of CT for T stage is 33% to 77% and 22% to 73% for nodal staging (2,3,4,5,6,7).
Endorectal ultrasound (EUS) is the most accurate preoperative staging tool for rectal cancer. Several studies have shown that the overall accuracy for T stage is 67% to 93% and for N stage is 61% to 88% (8,9,10,11,12,13,14,15,16,17,18,19,20). A very large study by Garcia-Aguilar et al. (21) reviewed the EUS experience in 1,184 patients with rectal tumors. Pathologic correlation with T stage was available in 545 patients and N stage in 238 patients. Ultrasound accuracy for T stage was 69%; 18% of tumors were overstaged and 13% understaged. With regard to nodal status, ultrasound accuracy was 64%. Thirty-two percent of the ultrasound N0 group were understaged and 48% of the N1 group were overstaged. Marusch et al. (22) reviewed the accuracy of EUS in determining the T-stage correlation in 422 rectal cancer resections. The overall accuracy was only 50.8% for T1 tumors and 58.6% for T2 lesions. The rate of understaging was 12.8% for the entire group (T1 to T4). Overstaging of the primary tumor is often attributed to peritumoral inflammation and hypervascularity, whereas understaging may be due to microscopic tumor invasion and technical problems with bulky tumors or lesions near the anal canal or valves of Houston (16,20,23,24). Lymph node overstaging may be due to reactive changes, whereas understaging can be due to the fact that nodal size may not correlate with the presence of metastases (20,25,26).
Magnetic resonance imaging (MRI) with endorectal coil may offer accuracy in T and N staging that rivals EUS. Bianchi et al. (27) reported that the diagnostic accuracy for phased array coil MRI was equal to EUS for T stage and nodal involvement. Kim et al. (28) recently reported impressive results with their use of MRI with phased array coils. The diagnostic accuracy of MRI with phased array coil was 97% for T1, 89% for T2, and 91% for T3 tumors, and the accuracy for the detection of nodal metastases was 95%.
The determination of the presence or absence of systemic disease includes conventional chest x-ray (CXR) to rule out pulmonary metastases. Abnormalities noted on CXR should be further investigated with a chest CT. Abdominal CT provides
valuable information with regard to the presence of metastatic hepatic disease, extrapelvic adenopathy, and the presence of peritoneal disease. Triple phase CT and MRI are used to fully delineate hepatic abnormalities that may be identified on initial abdominal-pelvic CT. Positron emission tomography (PET) scans play a valuable role in determining the presence of metastases and should be considered when there are equivocal findings on CT and/or MRI.
valuable information with regard to the presence of metastatic hepatic disease, extrapelvic adenopathy, and the presence of peritoneal disease. Triple phase CT and MRI are used to fully delineate hepatic abnormalities that may be identified on initial abdominal-pelvic CT. Positron emission tomography (PET) scans play a valuable role in determining the presence of metastases and should be considered when there are equivocal findings on CT and/or MRI.
Management of Locoregional Rectal Cancer
The goals of locoregional therapy for rectal cancer are complete tumor extirpation in a manner that maximizes survival and minimizes the risk of local recurrence yet preserves bowel continuity and the function of the anal sphincter, urinary bladder, and sexual organs. Due to the anatomic constraints of the pelvis, it is frequently difficult to attain all of these goals, and optimal treatment commonly requires a multidisciplinary approach. This approach involves a combination of chemoradiation therapy, surgery, and adjuvant chemotherapy. Adequate staging prior to institution of treatment is crucial to selecting optimal oncologic therapy for each patient. Treatment decisions must also include a careful risk/benefit discussion of aggressive cancer treatment with its associated morbidities of impaired bowel function, the need for permanent stoma, urinary and sexual dysfunction, and altered lifestyle.
In the past, rectal cancer was a surgical disease treated with resection alone, without attention to appropriate anatomic dissection and without knowledge of the modes of locoregional spread. This approach resulted in local recurrence rates around 30% and 5-year survival rates from 27% to 42% (29,30,31,32). In the past 20 years, the mechanism of locoregional spread has been elucidated, highlighting the importance of the mesorectum in rectal cancer resection. With the development of the technique of TME as a component of rectal cancer resection, there were marked improvements in both local recurrence rates as well as survival, with local recurrence rates dropping to below 10%, and 5-year survival rates improving to 70% or better (33,34). The recent advances in surgical technique and application of adjuvant and neoadjuvant therapy in the treatment of rectal cancer have made the management of this disease a true multidisciplinary endeavor. These recent advances in surgical technique, adjuvant therapy, and neoadjuvant therapy will be discussed.
The Mesorectum
The embryologic origin of the mesorectum is mesenchymal cells arranged in lamellae of rectal adventitia filled with fatty tissue during embryogenesis. The outer lamellae of the adventitia form the visceral fascia of the mesorectum. This visceral fascia envelopes the rectum and mesorectum (35,36). Parietal endopelvic fascia lines the pelvic walls, covering the piriformis, coccygeal, and levator ani muscles, the presacral venous plexus, the anterior surface of the sacrum and coccyx, and the anococcygeal ligament. The posterior visceral and parietal fascial layers are separated by an avascular plane of loose areolar tissue. This plane extends down to the anal sphincter. At the level of S4, the rectosacral fascia or ligament is encountered. This is a thickening of fibers between the visceral and parietal fascia that must be divided sharply to prevent traction injury and violation of the mesorectum or tearing of the presacral venous plexus. On the anterior surface, Denonvilliers’ fascia separates the rectum/mesorectum from the prostate and seminal vesicles in men and the posterior vaginal wall in women. Posterior to Denonvilliers’ fascia is the visceral fascia of the mesorectum. Dissection in the anterior plane can be performed anterior or posterior to Denonvilliers’ fascia depending on the location of the rectal tumor (37,38). Laterally, condensations of the visceral fascia known as the lateral ligaments are encountered. These ligaments connect to the parietal fascia and contain the middle rectal artery and autonomic nerves passing to the mesorectum/rectum. Division of the lateral ligaments in the appropriate plane prevents unnecessary injury to the pelvic autonomic nerve plexus (PANP) in the lateral pelvic sidewall.
Sympathetic and Parasympathetic Pelvic Nerves
The autonomic nervous system anatomy in the pelvis was described by Havenga et al. (39). Sympathetic nerve fibers enter the pelvis as the right and left hypogastric nerves, which originate from the superior hypogastric plexus located at the pelvic brim near the bifurcation of the aorta and vena cava. The nerves run in the areolar tissue plane between the visceral and parietal fascia of the mesorectum. Havenga et al. (39) demonstrated that the hypogastric nerves actually penetrated the visceral fascia for a short course near the pelvic brim. Other reports demonstrated that the nerves were posterior to the visceral fascia but closely associated with it (40,41,42). Given the close relationship between the hypogastric nerves and the visceral fascia of the mesorectum at this level, careful dissection in the areolar plane near the pelvic brim is required to identify and spare these nerves.
The parasympathetic nerve fibers (nervi erigentes) of the pelvis originate from the S2–S4 nerve roots. Their course runs under the parietal fascia over the piriformis muscle to the lateral pelvic sidewall. There, the parasympathetic fibers and the sympathetic fibers from the hypogastric nerves join to form the inferior hypogastric plexus or PANP. Rectal innervation from the PANP courses through the lateral ligaments to the rectum and mesorectum. Genitourinary branches of the PANP continue anteriorly under the parietal fascia to the bladder and to the prostate/seminal vesicles in men or the uterus/vagina in women (39). Excessive lateral dissection near the pelvic sidewall at the level of the lateral ligaments can injure the PANP and result in urinary and/or sexual dysfunction.
With a complete understanding of the relationships of the visceral fascia of the rectum/mesorectum, the parietal fascia of the pelvis, and the autonomic innervation of the pelvis, TME with autonomic nerve preservation (ANP) can be performed to mobilize the rectum and mesorectum for resection. Sharp dissection in the appropriate anatomic planes down to the level of the levators will facilitate maximal mobilization of the rectum for optimal sphincter preservation and distal margins and will minimize local recurrence and the sequelae of autonomic nerve dysfunction.
Technique of Total Mesorectal Excision and Complete Rectal Mobilization for Rectal Cancer
The modern methods of rectal cancer resection are based on complete mobilization of the rectum and mesorectum in the pelvis along appropriate anatomic planes with attention to preservation of the pelvic autonomic nervous system when possible. With adequate mobilization, resection margins can be optimized, chance of sphincter preservation can be maximized, and risk of local recurrence will be minimized.
Utilizing a low midline incision taken down to the pubis, the abdomen is explored for evidence of unsuspected metastatic disease. Retraction is established, and the sigmoid colon is exposed. The sigmoid and descending colon are mobilized lateral to medial, and the splenic flexure is taken down if a low pelvic reconstruction is anticipated. The left ureter and gonadal vessels are identified and are left in the retroperitoneum. With the sigmoid colon mobilized to midline, the peritoneum in the pelvis is opened along the visualized mesorectum circumferentially with cautery. Medially, the peritoneum is divided along the course of the superior rectal artery and vein to the level of the inferior mesenteric artery (IMA). If there is no palpable adenopathy at the level of the IMA, the vascular pedicle can be divided with preservation of the left colic branch and the bowel can be divided at the proximal sigmoid colon. If there is palpable adenopathy at the level of the IMA, the vascular pedicle should be divided at the level of the aorta, taking the left colic branch. The bowel in this case should be divided at the level of the distal descending colon. In this circumstance, the splenic flexure should always be mobilized if reconstruction is planned, and often the inferior mesenteric vein will need to be ligated at the inferior border of the pancreas to allow adequate mobilization of the descending colon for tension-free reconstruction. After division of the bowel and mesenteric vessels, the colon is packed in the left upper quadrant and attention is turned to the pelvic dissection.
Complete mobilization of the rectum and mesorectum begins with the posterior dissection. The rectosigmoid colon is lifted, and the retrorectal space is entered at the level of the pelvic brim immediately posterior to the superior rectal vessels. The areolar plane between the visceral mesorectal fascia and the parietal pelvic fascia is identified and divided sharply with cautery. The right and left hypogastric nerves course posterolaterally along the pelvic brim and are often attached to and lifted with the mesorectal fascia. These nerves should be identified and carefully dissected free of the visceral mesorectal fascia, and the dissection plane then continued medially to these nerves. Dissection posteriorly continues in the areolar plane to the level of S4 where the rectosacral fascia is encountered. This fascia is divided sharply with cautery or scissors to gain mobilization of the distal 5 cm of the rectal wall. Blunt dissection in this area can result in anterior tearing of the mesorectum with possible increased local recurrence rates or posterior tearing of the presacral venous plexus, resulting in difficult-to-control hemorrhage. Posterior dissection is complete with visualization of the levator ani musculature.
The anterior dissection in women begins with opening the peritoneum of the Pouch of Douglas. The uterus is lifted and the posterior wall of the vagina is identified. In women who have had a hysterectomy, identification of the posterior wall of the vagina can be facilitated by an assistant placing two fingers or a flat malleable retractor into the vagina from the perineum. Anterior dissection along the posterior wall of the vagina is performed sharply with scissors or cautery down to the level of the levators. In men, the dissection begins at the anterior cul-de-sac, identified by lifting the bladder superiorly with a retractor. Opening the anterior cul-de-sac will expose the seminal vesicles and the anterior surface of Denonvilliers’ fascia. For anterior tumors, continued dissection anterior to Denonvilliers’ fascia is performed with cautery or scissors down to the level of the levators. For other tumor location, similar dissection is performed posterior to this fascia.
After completion of the anterior and posterior dissections, the lateral sidewall dissection is completed. The lateral ligaments are identified and divided. At this location, the PANP should be identified and preserved if possible. If adequate lateral margin is possible, the lateral ligaments should be divided at the level of the visceral fascia of the mesorectum to minimize potential injury to the PANP. Division can be accomplished with cautery, clips, or stapling devices. With completion of the lateral sidewall dissection, the entire mesorectum and rectum are mobilized. At this point, if sphincter preservation is possible the rectum can be divided and the specimen removed. If sphincter preservation is not possible, the perineal portion of an abdominoperineal resection (APR) is undertaken to remove the specimen.
Extended Lymphadenectomy
In addition to TME, extended lymph node resection with ligation of the IMA at its junction with the aorta, and extended pelvic dissection and lymph node retrieval laterally along the pelvic sidewall have been advocated to improve local recurrence and survival. The issue of high ligation of the IMA is with regard to where the vascular pedicle is divided. Traditional ligation of the vascular pedicle is performed just distal to the origin of the left colic artery. However, anatomic studies have revealed that as many as 10 lymph nodes could be found between the origin of the left colic vessel and the origin of the IMA (43). In addition, pathology reviews reported that the lymph nodes between the origin of the IMA and the left colic branch were positive in 11% to 22% of cases (44,45). Therefore, high ligation of the IMA was proposed as a method to improve resection and survival. However, subsequent reports failed to support the superiority of high ligation, and it is not routinely practiced for oncologic reasons (46,47,48).
Lateral pelvic lymph nodes have been reported to be involved in rectal cancer in 9% to 14% of patients (49,50,51). Extended lateral lymph node dissection has been proposed by several Japanese groups to provide decreased local recurrence and improved survival (52). However, reported rates of local control have been variable, and overall survival rates are similar to those reported for TME alone. In addition, this extended lymphadenectomy is consistently associated with high rates of urinary and sexual dysfunction (53,54,55). With the routine use of neoadjuvant and adjuvant therapy in Western medicine, there is no current role for prophylactic lateral lymph node dissection in the treatment of rectal cancer.
Rectal Cancer Resection
The goal of resection in rectal cancer is complete tumor removal with negative circumferential (radial) and distal margins with en bloc resection of involved pelvic organs if necessary. Complete mobilization of the mesorectum down to the level of the levator ani complex using techniques describe above is crucial to attaining this goal and minimizing local recurrence rates. Secondary goals in rectal cancer resection include preserving the anal sphincter, re-establishing bowel continuity, preserving bladder and sexual function, and restoring the reservoir function of the rectum when indicated. Maximal achievement of these secondary goals is dependent on adequate knowledge of the appropriate planes of dissection in the pelvis and complete mobilization of the rectum and mesorectum.
Sphincter Preservation Procedures
The anal sphincter complex cannot be preserved in cases in which the cancer directly invades the external sphincter/levator ani complex and in cases where a negative distal margin cannot be achieved with a continent sphincter. In all other cases, the possibility of sphincter preservation and re-establishment of bowel continuity remains an option. However, the final decision can only be made intra-operatively, after complete rectal/mesorectal mobilization is performed and the tumor mobilized.
Only then can the distal margin and radial clearance be assessed and the likelihood of re-establishing bowel continuity be evaluated.
Only then can the distal margin and radial clearance be assessed and the likelihood of re-establishing bowel continuity be evaluated.
Low Anterior Resection
The hallmarks of low anterior resection for rectal cancer are complete mobilization of the rectum/mesorectum with division of the lateral ligaments and an extraperitoneal pelvic anastomosis. Tumors of the upper and middle rectum are treated most commonly with this procedure. Lower rectal lesions may be amenable to low anterior resection with a very low anastomosis depending on the size and location of the tumor, the ability to completely mobilize the rectum, and the ability to divide the rectum distal to the lesion with negative margins from an abdominal approach.
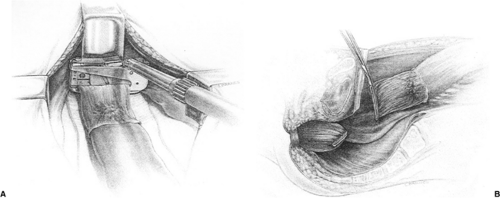
For lesions of the upper and middle rectum, the rectum is completely mobilized to the pelvic floor using the TME techniques described earlier. After mobilization, the rectum is divided with a transanastomotic (TA) stapling device, using a bowel clamp on the proximal margin (Fig. 44.1). There is some debate as to the extent of distal dissection/resection for upper rectal lesions. Distal margin length is rarely an issue for these lesions. Heald (33) advocates total mesorectal resection down to the pelvic floor for virtually all rectal cancers. Some researchers advocate complete mobilization of the rectum to the pelvic floor with division of the rectum 5 cm beyond the lower margin of the tumor (34,56,57). Although this may result in incomplete resection of the entire mesorectum for upper rectal lesions, it does not compromise oncologic outcome, as pathologic studies have demonstrated no distal spread of cancer in the mesorectum beyond 5 cm (50,57). A key component to the oncologic outcome of the 5-cm margin is that the mesorectum must be divided at right angles to the wall of the rectum to ensure adequate mesorectal resection. There is a tendency to cone down to the bowel wall distal to the lesion, which can potentially leave distal disease in an incompletely resected mesorectum. If there is any question regarding the margin, frozen section analysis is performed. After assurance of adequate distal margins, bowel continuity is restored using a side-to-end anastomosis, hand sewn or stapled (Fig. 44.2), or an end-to-end stapled anastomosis using an end-to-end anastomosis (EEA) stapling device (Fig. 44.3). For lesions in the upper and middle rectum, there is a sufficient distal rectal remnant for reservoir function, so reservoir construction in the proximal bowel with J-pouch or coloplasty is not required. Proximal diversion with a loop ileostomy is not required with upper and middle rectal reconstruction; however, in the setting of neoadjuvant chemoradiation, liberal use of proximal diversion is encouraged.
Tumors in the distal rectum can also be resected with low anterior resection and a very low rectal anastomosis. The rectum is mobilized to the pelvic floor with complete mobilization of the tumor. A distal margin of 2 cm is the goal of the resection. The rectum is divided just above the pelvic floor with a TA stapling device leaving only a small cuff of rectum above the anal canal. The specimen is opened and the distal margin assessed. If the margin is <2 cm, frozen section analysis is performed to ensure a negative margin. If the margin is positive or close, resection of the remainder of the rectum and coloanal reconstruction may be required. After confirming adequate distal margins, bowel continuity is restored with an end-to-end stapled anastomosis with an EEA stapling device. With very low rectal anastomoses, there is no significant rectal remnant to function as a reservoir. Reconstruction with a J-pouch or coloplasty should be considered for improved functional outcome (see below). We encourage proximal diversion of all low rectal reconstructions, especially in the setting of neoadjuvant chemoradiation and/or J-pouch or coloplasty reconstruction.
Proctectomy with Coloanal Anastomosis
For lesions of the distal rectum that do not involve the levator ani complex but cannot be resected to negative distal margins from an abdominal approach, proctectomy with coloanal reconstruction can allow sphincter preservation in selected patients. After complete mobilization of the rectum to the pelvic floor using the principles of TME, the distal margin is assessed. If there is insufficient room in the pelvis to divide the rectum with a negative distal margin, division of the rectum from a perineal approach via the anal canal is undertaken. For this approach, the anal canal is everted with several heavy sutures. Cautery is used to open the mucosa just above the dentate line
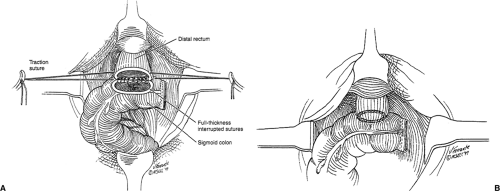
down through the internal sphincter. Dissection is then performed in the intersphincteric plane up to the pelvis in a circumferential fashion, completely freeing the rectum. The specimen is sent to pathology for both distal and radial margin frozen section. If the margins are positive, APR is performed. If margins are adequate, bowel continuity is restored with a hand-sewn anastomosis at the level of the dentate line (Fig. 44.4). Reconstruction can be done with a straight coloanal anastomosis or with reservoir construction with a J-pouch or coloplasty. Proximal diversion with a loop ileostomy is then performed.
down through the internal sphincter. Dissection is then performed in the intersphincteric plane up to the pelvis in a circumferential fashion, completely freeing the rectum. The specimen is sent to pathology for both distal and radial margin frozen section. If the margins are positive, APR is performed. If margins are adequate, bowel continuity is restored with a hand-sewn anastomosis at the level of the dentate line (Fig. 44.4). Reconstruction can be done with a straight coloanal anastomosis or with reservoir construction with a J-pouch or coloplasty. Proximal diversion with a loop ileostomy is then performed.
Pouch Reconstruction
Very low rectal anastomoses and coloanal anastomoses can significantly alter defecation patterns in the postoperative period. Frequency, urgency, soiling, and incontinence are all increased (58). The sensations of frequency and urgency are likely due to loss of the rectal reservoir with resection (59,60). Frequency of stool has been correlated to length of residual rectum, with increasing stool frequency associated with shorter rectal remnants (61). Continence is also affected by low pelvic or coloanal anastomoses. Sphincter resting pressures are significantly reduced with coloanal anastomoses (62). Continence can also be affected by the use of transanal stapling devices and reduction in anal sensation (63,64). Over time, frequency, urgency, and continence can improve, and the improvement is likely due to dilation of the colon above the anastomosis to allow better reservoir function (65,66).
Efforts to improve functional outcome of low anastomoses focused on construction of a pouch in the colon just proximal to the anastomosis to improve the reservoir function of the neorectum. The two methods of pouch construction are J-pouch and coloplasty. Formation of either pouch construction requires complete mobilization of the left colon with takedown of the splenic flexure, ligation of the IMA at its origin, and division of the inferior mesenteric vein at the inferior boarder of the pancreas to allow sufficient length for a tension-free anastomosis in the pelvis. Formation of the J-pouch begins with folding the end of the colon back on itself for a length of 5 to 7 cm. A colotomy is made in the anterior wall of the folded colon at the site of planned anastomosis, and a gastrointestinal anastomosis (GIA) stapler is placed into the opening and across the posterior wall. The stapler is fired, creating the pouch with an ideal size of 5 to 7 cm (67). The anastomosis is then completed using a hand-sewn or stapled technique (Fig. 44.5). The coloplasty pouch is formed in the manner of a Heinecke–Mikulicz strictureplasty. At 4 to 6 cm proximal to the end of the colon, an 8- to 10-cm longitudinal colotomy is made. The colotomy is then closed in a transverse fashion, creating the pouch (Fig. 44.6). The anastomosis is completed at the distal end of the colon using a hand-sewn or stapled technique.
Colonic J-pouch reconstruction was first reported in 1986 by Lazorthes et al. (68) and Parc et al. (69). Both reports found improvements in function with the construction of a colonic reservoir as reflected by decreased frequency of stool, decreased urgency, and decreased incontinence. Several randomized trials comparing J-pouch to straight anastomosis have also been performed, demonstrating improved function with pouch reconstruction. Ortiz et al. (70) and Ho and Seow-Choen (71) demonstrated significant improvements in stool frequency at 12 months with J-pouch reconstruction.
Seow-Choen and Goh (72) found significant improvement in both frequency of stool and continence at 12 months after reconstruction. Hallbook et al. (73) showed J-pouch reconstruction was significantly better for frequency, urgency, and continence at 12 months. Long-term studies comparing J-pouch reconstruction to straight anastomosis report durable improvements in stool frequency at 24 months and 60 months with the J-pouch (74,75). Improvements in urgency and continence are no longer evident at 24 months (74,75,76). An additional technical benefit of J-pouch reconstruction is an apparent lower rate of anastomotic leak, likely a result of improved perfusion of the J-pouch at the anastomosis site (73,77,78).
For patients who cannot undergo J-pouch reconstruction due to fatty colon, diverticular disease, small pelvis, or inadequate colon length to reach the distal pelvis with a J-pouch, coloplasty pouch formation can be utilized (79,80). Functional outcomes with coloplasty appear similar to those of J-pouch reconstruction. A prospective randomized trial comparing coloplasty to J-pouch reconstruction has completed accrual and is in the follow-up phase (58).
APR
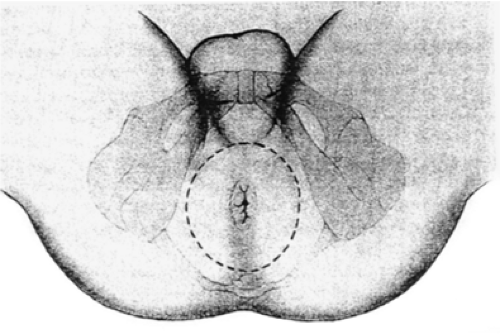
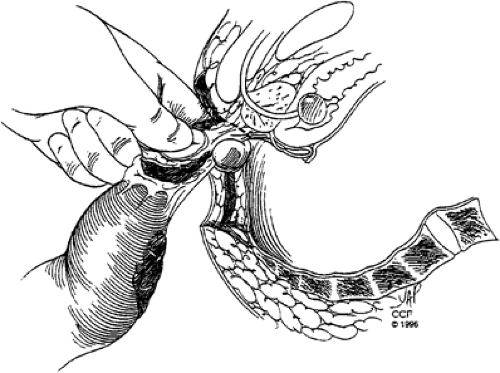
For patients with low rectal tumors directly invading the levator ani complex or tumors that cannot be resected with negative circumferential and/or distal margins with preservation of the anal sphincter complex, APR with permanent end colostomy is required. APR and sphincter-saving procedures appear to have similar oncologic outcomes when comparing tumors of similar stage, pathologic features, and distance from the anal verge (81,82,83,84,85). The abdominal portion of the procedure is performed similar to that of low anterior resection, with complete mobilization of the rectum using the principles of TME. After mobilization of the rectum and confirmation of the need for sphincter resection, the perineal portion of the operation is performed. With the patient positioned in the lithotomy position in stirrups, an elliptical incision is made from the midpoint of the perineal body anteriorly to the level of the coccyx posteriorly encompassing the anus and 2 to 3 cm of skin measured for the anal verge (Fig. 44.7). In women with low-lying tumors involving the anterior surface of the rectum, posterior vaginectomy should be performed for adequate circumferential margins (Fig. 44.8). Cautery dissection is used to open the posterior and lateral tissues to the level of the pelvic floor. The inferior hemorrhoidal arteries are encountered in the ischiorectal space and should be ligated.
The coccyx is then palpated, and the
anococcygeal ligament is divided anterior to the tip of the coccyx. Dissection in this posterior plane is continued superiorly until the posterior pelvic floor is opened. This dissection can be facilitated by the abdominal surgeon placing a hand in the deep posterior pelvis for guidance. Next, the lateral levator ani complex is divided on both sides from a posterior to anterior approach (Fig. 44.9). The specimen is then delivered through the perineal wound, and the anterior dissection is completed. In men, careful palpation of the prostate and prostatic urethra during the anterior dissection is critical to maintaining the correct anatomic plane and preventing prostatic and/or urethral injury (Fig. 44.10). In women, the appropriate plane is along the posterior wall of the vagina. This plane may be scarred secondary to complications of childbirth and may necessitate resection of a small portion of the vaginal wall at the introitus. With anterior or circumferential tumors, larger resection of the posterior vaginal wall is required. The posterior wall of the vagina is resected between clamps. The cut edge is highly vascular and often requires suture ligation of bleeding vessels after the specimen is delivered. After margin analysis to assure complete resection, the perineum is irrigated and drains are placed in the pelvis. The skin is closed with sutures or staples. For women with posterior vaginal wall resection, the posterior wall can be closed primarily with the perineal wound in cases with small defects.
anococcygeal ligament is divided anterior to the tip of the coccyx. Dissection in this posterior plane is continued superiorly until the posterior pelvic floor is opened. This dissection can be facilitated by the abdominal surgeon placing a hand in the deep posterior pelvis for guidance. Next, the lateral levator ani complex is divided on both sides from a posterior to anterior approach (Fig. 44.9). The specimen is then delivered through the perineal wound, and the anterior dissection is completed. In men, careful palpation of the prostate and prostatic urethra during the anterior dissection is critical to maintaining the correct anatomic plane and preventing prostatic and/or urethral injury (Fig. 44.10). In women, the appropriate plane is along the posterior wall of the vagina. This plane may be scarred secondary to complications of childbirth and may necessitate resection of a small portion of the vaginal wall at the introitus. With anterior or circumferential tumors, larger resection of the posterior vaginal wall is required. The posterior wall of the vagina is resected between clamps. The cut edge is highly vascular and often requires suture ligation of bleeding vessels after the specimen is delivered. After margin analysis to assure complete resection, the perineum is irrigated and drains are placed in the pelvis. The skin is closed with sutures or staples. For women with posterior vaginal wall resection, the posterior wall can be closed primarily with the perineal wound in cases with small defects.
The perineal wound remains a major source of postoperative complications after APR. Wound complications can occur in 20% to 60% of cases (85,86,87). For patients with large tissue defects, prior pelvic irradiation, or extensive vaginal resection with APR, perineal reconstruction with a myocutaneous flap can be used to facilitate reconstruction and decrease wound morbidity. The rectus abdominis musculocutaneous (RAM) flap has been used in this situation with excellent results, providing a well vascularized, mobile flap to fill the soft tissue defect, repair the vaginal defect if necessary, and decrease the incidence of perineal wound complications (85,86,87,88,89). Early involvement of the plastic surgery reconstruction team in operative planning prior to APR is critical to facilitate optimal perineal closure and improve outcome.
Urinary and Sexual Dysfunction After TME
The prevalence of urinary dysfunction after TME with attention to preservation of the autonomic nerve plexi is markedly improved as compared to that after conventional rectal surgery. The incidence of urinary dysfunction after conventional rectal resection ranges from 8% to 70% (90,91,92,93). With TME techniques with attention to preservation of the autonomic nerves in the pelvis, this incidence has been significantly improved. Urinary dysfunction in recent reports using TME techniques with ANP have ranged from 0% to 6.6% (39,93,94). In patients with urinary dysfunction immediately after surgery, function appears to improve with time (39,93).
Sexual dysfunction after conventional rectal surgery was a common occurrence, with incidence rates reported from 37% to 68% of patients (95,96,97,98). TME with ANP led to marked improvements in outcome for sexual function. With TME and ANP, the ability to have intercourse was preserved in 86% of patients younger than 60 years and in 67% of patients older than 60 years or undergoing APR. Orgasm could be achieved in 87% of men and 91% of women (39). In a small prospective study, TME with ANP was demonstrated to cause no impairment in sexual function (99). Masui et al. (100) demonstrated that the degree of sexual function impairment in 134 sexually active men depended on the degree of ANP with TME. Patients were grouped into three categories: bilateral complete nerve preservation, unilateral complete nerve preservation, and pelvic nerve plexus preservation only. For patients with bilateral complete nerve preservation, erection was preserved for 93%, erection sufficient for vaginal insertion was preserved in 90%, ejaculation was preserved in 83%, and orgasm was preserved in 94%. For patients with unilateral complete nerve preservation, the results were 82%, 53%, 47%, and 65% for the respective categories. For patients with pelvic plexus only preservation, the results were 61%, 26%, 0%, and 22%,
respectively. Although preservation of sexual function is an important goal, it should not compromise the oncologic outcome. In many cases, complete ANP may not be possible secondary to tumor size, location, and anatomic constraints in the pelvis. In all cases of pelvic surgery, the patient must be counseled regarding the risks of sexual and urinary dysfunction that can occur. Postoperative sexual function should also be evaluated and followed, as patients seldom discuss or receive treatment for sexual dysfunction after rectal cancer therapy (101).
respectively. Although preservation of sexual function is an important goal, it should not compromise the oncologic outcome. In many cases, complete ANP may not be possible secondary to tumor size, location, and anatomic constraints in the pelvis. In all cases of pelvic surgery, the patient must be counseled regarding the risks of sexual and urinary dysfunction that can occur. Postoperative sexual function should also be evaluated and followed, as patients seldom discuss or receive treatment for sexual dysfunction after rectal cancer therapy (101).
TAE
Although transabdominal resection using TME principles remains the gold standard for surgical therapy for rectal cancer, this approach can result in significant morbidity to the patient with regard to urinary dysfunction, sexual dysfunction, altered bowel function, risk of anastomotic leak, continence, and need for permanent stoma. The transabdominal approach also represents a significant physiological stress that would not be tolerated in patients with significant medical comorbidities. Efforts to minimize morbidity, offer treatment to patients with severe comorbidities, and treat patients who refused standard resection focused on local resection of rectal cancer using a transanal approach. With reasonable local control rates in these populations, the local excision approach was applied to patients who were otherwise fit and willing to undergo transabdominal resection. The question of whether local excision is an oncologic equivalent to transabdominal resection based on TME principles in appropriately selected patients is still under scrutiny.
Appropriate patient selection is the key to optimizing outcomes from a local resection approach. Because local excision does not address lymph node spread, patient selection hinges on identification of patients with no lymph node metastases. The diagnostic accuracy of predicting nodal involvement with preoperative imaging has been discussed elsewhere. In addition, there are multiple clinical and pathologic tumor characteristics that are associated with lymph node metastases in rectal cancer. Depth of invasion into the rectal wall is a significant predictor for presence of nodal disease, with an incidence of nodal metastases in T1 lesions from 0% to 13%, T2 lesions 12% to 28%, T3 lesions 33% to 66%, and T4 lesions 53% to 79% (102,103,104,105,106). Additional predictors reported include tumor differentiation and presence of lymphovascular invasion, mucinous features, and tumor ulceration (107). Given that T1 lesions have the lowest incidence of lymph node involvement and should be the optimal targets for local excision, studies have focused on these lesions to identify the incidence and predictors of nodal spread. Kikuchi et al. (108) divided T1 lesions by depth of invasion into the submucosa with slight invasion of the muscularis mucosa termed sm1, intermediate invasion termed sm2, and invasion extending to the inner surface of the muscularis propria termed sm3. In their series, there was no incidence of lymph node involvement for sm1 lesions but a 25% incidence of lymph node involvement for sm3 lesions. Nascimbeni et al. (109) reviewed data on 359 patients with resected T1 lesions of the colon or rectum and found a 13% overall incidence of lymph node metastases, with deep invasion into the submucosa (23% incidence of nodal disease), lymphovascular invasion (32% incidence of nodal disease), and location of the tumor in the distal one third of the rectum (34% incidence of nodal disease) as predictors of nodal involvement on multivariate analysis. Tumor grade was not significant on multivariate analysis. As demonstrated above, even T1 lesions have a marked variability in the incidence of lymph node metastases that would predict oncologic failure of local excision alone.
Other factors to be considered for local excision include tumor size, with lesions larger than 3 to 4 cm having a higher rate of local recurrence (110,111,112). Technical aspects of resection from a transanal approach often exclude tumors with involvement of >40% of the circumference of the rectal wall and tumors >10 cm from the anal verge. Based on tumor factors with regard to nodal spread and technical limitations of TAE, current recommendations for consideration for TAE of rectal cancer include tumor–node–metastasis (TNM) stage T1 or T2 N0 tumors with low grade histology, no evidence of lymphovascular invasion, size smaller than 4 cm, involvement of <40% of the rectal wall circumference, and position <10 cm from the anal verge.
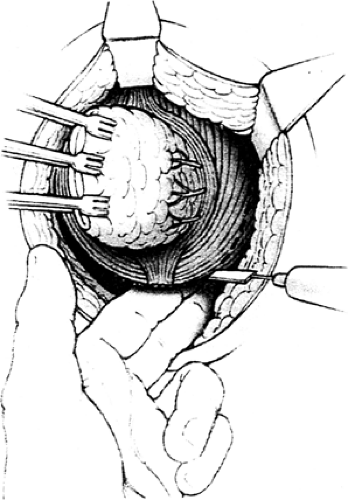
The technical aspects of TAE are straightforward. All patients receive preoperative bowel prep and IV antibiotics. General or regional anesthesia can be used as long as complete paralysis of the anal sphincter can be accomplished. The patient is positioned in lithotomy for posterior lesions or in the prone-jackknife position for anterior lesions. After adequate anal dilation and establishment of retraction, the lesion is identified and a 1- to 2-cm margin is demarcated with cautery. A full-thickness excision with cautery is then performed down to perirectal fat. Care must be taken with anterior lesions to prevent injury to the prostate or posterior vaginal wall. The excised specimen is then carefully oriented and sent to pathology for margin analysis. Any close margin should be checked with frozen section, and if possible, additional margin should be taken to obtain a 1-cm margin around the lesion. After margins are verified, the defect in the rectal wall is closed with absorbable sutures. Patients are generally discharged within 24 to 48 hours after the procedure. Reported complications range from 0% to 22%, with bleeding, local sepsis, urinary infection or retention, anal incontinence, and rectovaginal fistula being the most common (106,107).
The results of TAE vary in numerous reports of single-institution results as there are no randomized controlled trial data to examine. Many of the early experiences with TAE alone were reviewed by Sengupta and Tjandra in 2001 (107). In this study, 22 trials with a total of 958 patients were examined, and local recurrence rates by T stage were reported. The overall local recurrence rate was 14%; for T1 lesions there was a 10% local recurrence rate (range 0% to 24%), for T2 lesions a 25% local recurrence rate (range 0% to 50%), and for T3 lesions a 38% local recurrence rate (range 0% to 100%) (106). These local recurrence rates were higher than would be expected with standard transabdominal resection based on TME principles. Mellgren et al. (104) compared local excision to RAD in a retrospective review in which neither group of patients received adjuvant chemoradiation. The groups compared included patients with 108 local excisions with 4.4 years of follow-up and 153 RADs with 4.8 years of follow-up. The groups differed significantly in distance of the lesion from the anal verge (lower in the local excision group), tumor size (smaller in the local excision group), and T stage (36% T2 for local excision vs 78% T2 for RAD). The results of the comparison showed a higher rate of local recurrence after local excision as compared to RAD (T1 = 18% vs 0%, T2 = 47% vs 6%) as well as a higher rate of overall recurrence (T1 = 21% vs 9%, T2 = 47% vs 16%). Although there was no difference in the 5-year cancer-specific mortality between the two groups, there was a significantly lower 5-year overall survival rate for T2 lesions treated with local excision as compared to RAD (65% vs 81%). The results of this comparison clearly suggest that T2 rectal cancers treated with local excision alone have inferior outcomes as compared to radical surgery. The results are less clear for T1 lesions from this study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree