Pancreas Cancer: Anatomy, Staging Systems, and Techniques
Kevin Conlon
M. A. Aremu
Introduction
In clinical practice, pancreatic cancer is often used synonymously with pancreatic ductal adenocarcinoma, which constitutes more than 90% of all primary malignant tumors arising from the exocrine portion of the gland. Pancreatic cancer remains a highly lethal disease; on a worldwide basis, it affects as many as 545,000 patients per year (1) with a death–incidence ratio of 0.99% (2). In the United States, it ranks fourth in males and fifth in females as a leading cause of death behind lung, breast, prostate, colorectal, and ovarian cancer, and the disease now accounts for 10% of all cancers of the digestive tract, second behind colorectal cancer (3). Surgical resection remains the only potentially curative intervention for those patients with localized disease, yet only one in five patients will be resectable at presentation; the remainder will have locally advanced or metastatic disease (4).
Hence, precise staging of this disease is important to enable accurate patient stratification and assessment of response to therapy, as well as to avoid unnecessary morbidity and mortality and diminished quality of life in this population of patients with very poor prognosis (5). Staging should accurately define the extent of disease, direct appropriate therapy, and avoid unnecessary intervention in a safe and cost-efficient fashion.
Anatomy of the Pancreas
The pancreas is a retroperitoneal, lobulated, yellow organ that lies within the “C” curve of the first, second, and third parts of the duodenum and extends transversely in front of the inferior vena cava and aorta and behind the stomach to the hilum of the spleen. In adults, it measures between 12 and 15 cm in length and weighs about 100 g in men and 85 g in women (6).
Based on anatomical relations, the pancreas is divided into head, neck, body, and tail. The uncinate process is an accessory lobe that extends from the head of the gland lying posterior to the superior mesenteric vessels. The head is the broadest and thickest part and fits into the “C” curve of the duodenum, lying over the inferior vena cava. The neck joins the head and the body, and it is marked anteriorly by the gastroduodenal artery groove and posteriorly by the commencement of the portal vein. The body, which is the longest part of the gland, runs from the neck to the left toward the tail of the pancreas. The tail is the narrowest portion of the gland, and it extends to the hilum of the spleen (Fig. 26.1) (7).
Embryologically, the pancreas develops from two buds—the ventral and dorsal buds—which are outgrowths of the endoderm at the junction of foregut and midgut. The dorsal bud develops into the tail, body, neck, and part of the head, while the ventral bud gives rise to the rest of the head and the uncinate process. The normal rotation of the gut leads to the ventral bud rotating dorsally to fuse with the dorsal bud to form a single adult gland. Fusion of the ventral bud to the dorsal bud both posteriorly and anteriorly gives rise to annular pancreas in which a ring of pancreatic tissue encircles the second part of the duodenum, causing duodenal obstruction.
The pancreas is both an exocrine and an endocrine organ. Acini cells and ducts develop from endodermal tubules within both pancreatic buds, and secrete various digestive enzymes and bicarbonate (exocrine function). The endocrine function is by the islets cells, which are isolated clumps of endodermal cells derived from the tubules and comprises of alpha cells (produce glucagon), beta cells (produce insulin), delta cells (produce somatostatin), and PP cells (produce pancreatic polypeptides).
The exocrine secretions of the gland is drained by the main pancreatic duct (duct of Wirsung), which runs from the tail toward the head of pancreas and drains into the second part of the duodenum at the major papillae by joining with the common bile duct to form the ampulla of Vater. The main duct is formed by the fusion of the distal two-thirds of the dorsal duct and the ventral pancreatic duct. Persistence of the proximal one-third of the dorsal pancreatic duct gives rise to the accessory pancreatic duct (duct of Santorini), which drains the inferior part of the head and the uncinate process. The accessory duct empties into the duodenum via the minor papilla, which is located about 2 cm anterosuperior to the major papilla, or it may end blindly with some connecting channels to the main duct.
Pancreatic divisum is a congenital abnormality found in roughly 10% of the population in which the dorsal and ventral pancreatic ducts do not fuse. This results in the dorsal pancreatic duct, which drains the head, neck, body, and tail of the gland emptying into the duodenum via the minor papilla (predisposing to recurrent pancreatitis), and the ventral pancreatic duct, which drains the inferior portion of the head of pancreas and the uncinate process, emptying via the major papilla.
The pancreas receives the following main blood supply: the anterior and posterior superior pancreaticoduodenal arteries (from the gastroduodenal artery) supply the head, uncinate process, and the duodenum, together with anterior and posterior inferior pancreaticoduodenal arteries (from the superior mesenteric artery or its first jejunal branch). Many small branches from the splenic artery supply the neck, body, and tail of the pancreas, including arterial pancreatica magna (to the
body) and arteria cauda pancreatica (to the tail). Considerable variations in the blood supply to the pancreas have been described (8,9). The venous drainage of the head and neck is via the superior and inferior pancreaticoduodenal veins, and the body and tail drain by numerous small veins into the splenic vein; hence, the venous return is primarily into the portal system.
body) and arteria cauda pancreatica (to the tail). Considerable variations in the blood supply to the pancreas have been described (8,9). The venous drainage of the head and neck is via the superior and inferior pancreaticoduodenal veins, and the body and tail drain by numerous small veins into the splenic vein; hence, the venous return is primarily into the portal system.
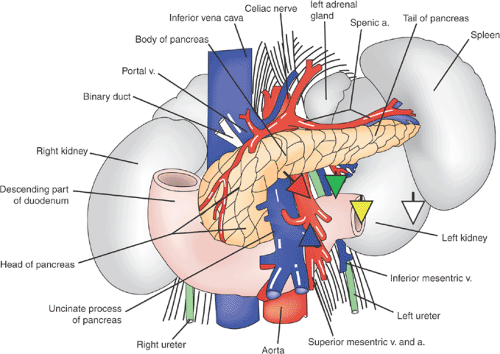 FIGURE 26.1. Anatomy of the pancreas and related structures. Source: Adapted from ref. 7 (See also color Figure 26.1). |
Lymphatic vessels from the pancreas follow the course of the arteries. The body and tail drain into the pancreaticosplenic nodes along the splenic artery, and the neck and head drain into pancreaticoduodenal, superior mesenteric, hepatic, and celiac lymph nodes. There is some direct drainage from the pancreas to the periaortic lymph nodes (10).
The innervation of the pancreas is by both sympathetic and parasympathetic systems. The sympathetic nerve supply, which regulates pancreatic blood flow by vasoconstriction, comes from T6 to T10 segments of the spinal cord via splanchnic nerves and the celiac plexus. Parasympathetic innervation stimulates exocrine secretions and is derived from the posterior vagus nerve and the celiac plexus. Sensory fibers, including pain fibers, are carried by both the sympathetic and parasympathetic fibers.
Staging Systems
In the United States, the most widely used staging system for pancreatic cancer is that developed by the American Joint Committee on Cancer in cooperation with the TMN Committee of the International Union Against Cancer. This classification represents an expression of the anatomical extent of the disease, taking into account the size and invasiveness of the primary tumor (T), the presence or absence of regional nodal metastases (N), and the existence or nonexistence of distant metastatic disease (M). Table 26.1 illustrates the components of this system as it relates to pancreatic cancer. Histologic grade, although shown to have prognostic significance in some studies (11), is not included in the current classification. For pancreatic cancer, in particular, local extension of the tumor may lead to unresectability. This is reflected in the staging system because T3 tumors are those that do not involve unresectable structures and T4 tumors do (Table 26.1).
Diagnosis and Staging
Clinical Presentation
Sener et al., reviewing the National Cancer Database, identified more than 100,000 patients diagnosed with pancreatic cancer between 1985 and 1995 (12). The head of the gland was the predominant site of disease (78%), while the body was the site in 11% and the tail in 11% of patients. Others have confirmed this distribution of disease (13,14,15). Sener et al. also noted that the ratio of limited (stage I) to advanced disease (stage IV) was 0.70 for lesions in the head of the gland, 0.24 for body tumors, and 0.10 for tail tumors (12).
Delayed presentation has been proposed as the reason for this apparent stage imbalance rather than inherent biological differences. Unlike tumors situated in the head of the gland in which obstructive jaundice may produce symptoms and signs, lesions of the pancreatic body and tail usually present with prolonged, nonspecific symptoms. Gastrointestinal symptoms such as nausea, anorexia, early satiety, and alteration in bowel function are common. These often prompt unsuccessful, empiric therapies that contribute to the delayed presentation.
The classic triad of abdominal pain, weight loss, and jaundice has generally been associated with pancreatic cancer. Modolell et al. (16) found abdominal pain to be the most
common presenting complaint, whereas Maringhini et al. (17) reported weight loss and elevated bilirubin and alkaline phosphatase to have a negative predictive value of 95%. Nix et al. analyzing 123 patients with a carcinoma at the head of the pancreas found the frequencies of the classic triad and other symptoms to be abdominal pain (70.7%), weight loss (80.5%), jaundice (88.6%), tiredness and malaise (42.3%), change in bowel habits (41.5%), sudden onset of diabetes mellitus (33.3%), and upper abdominal discomfort (22.0%). However, they noted that jaundice and abdominal pain are late symptoms (18).
common presenting complaint, whereas Maringhini et al. (17) reported weight loss and elevated bilirubin and alkaline phosphatase to have a negative predictive value of 95%. Nix et al. analyzing 123 patients with a carcinoma at the head of the pancreas found the frequencies of the classic triad and other symptoms to be abdominal pain (70.7%), weight loss (80.5%), jaundice (88.6%), tiredness and malaise (42.3%), change in bowel habits (41.5%), sudden onset of diabetes mellitus (33.3%), and upper abdominal discomfort (22.0%). However, they noted that jaundice and abdominal pain are late symptoms (18).
Table 26.1 American Joint Committee on Cancer Staging Classification for Exocrine Pancreatic Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dalton et al., reviewing the Mayo Clinic’s experience, noted that the majority of their patients (92%) were symptomatic at presentation with the median duration of symptoms being 6 months (19). Generally, the symptoms were vague and nonspecific, with abdominal or back pain and weight loss predominating. Others have reported similar results (20). Physical signs are often lacking with <25% presenting with a palpable mass or ascites and less than one-third of patients have palpable gallbladder (21) (Courvoisier sign).
Laboratory investigations such as serum amylase or liver function tests are usually normal, except in obstructive jaundice or in association with liver metastases. Serum tumor markers such as CA 19-9 may be elevated but are not sensitive or specific enough to differentiate adenocarcinoma of the pancreas from benign pancreatic pathology or other gastrointestinal cancers. However, studies (22,23) have shown that >300 U/mL is associated with advanced pancreatic cancer and that a threshold level of 150 U/mL has a specificity, sensitivity, and positive predictive value of 90%, 59%, and 88%, respectively, for predicting unresectability (24).
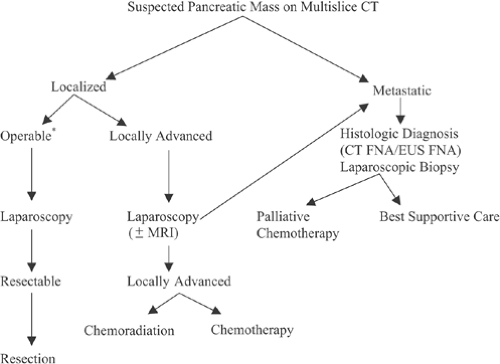
The goal of clinical staging for patients with pancreatic cancer is the identification of the subset of patients who would be candidates for curative surgical resection. Historically, surgical exploration has been the gold standard for determination of resectability. However, our current algorithm (Fig. 26.2) suggests that exploration be reserved for those patients considered to be resectable following laparoscopic staging or those patients in whom operative palliation is required. In the following discussion, we present the clinical staging modalities currently available, as well as their strengths and weaknesses, and relate our approach to the staging of patients with a pancreatic mass.
Before the advent of cross-sectional imaging, percutaneous cholangiography (PTC), and endoscopy, the workup of patients with painless jaundice, abdominal pain, or an abdominal mass was straightforward. An exploratory laparotomy would be performed for identification of the primary pathology and assessment of resectability.
Resection of the primary tumor with negative margins (RO resection) remains the aim of the operating surgeon. In general, at exploration, the surgeon proceeds with visualization of the peritoneal surfaces, palpation of the liver, palpation of the retroperitoneal nodes, and dissection of the blood vessels. The primary tumor is also assessed. Tumors are considered unresectable by virtue of distant disease (peritoneal, nodal, or liver metastases) or local invasion involving the superior
mesenteric artery, hepatic artery, or celiac trunk. Long segment involvement of the portal vein or its tributaries is also considered by most to be a barrier to resection. Invasion of the tumor into the stomach, colon, or duodenum is not in itself a contraindication to resection because these organs can be taken en bloc with the primary tumor if the vessels are free. However, in many cases, contiguous organ involvement also indicates vascular encasement. Lymphadenopathy located in the porta hepatis, the root of the transverse mesocolon, or the celiac region is considered metastatic, and although resectable, the patient is considered to have stage IV disease.
mesenteric artery, hepatic artery, or celiac trunk. Long segment involvement of the portal vein or its tributaries is also considered by most to be a barrier to resection. Invasion of the tumor into the stomach, colon, or duodenum is not in itself a contraindication to resection because these organs can be taken en bloc with the primary tumor if the vessels are free. However, in many cases, contiguous organ involvement also indicates vascular encasement. Lymphadenopathy located in the porta hepatis, the root of the transverse mesocolon, or the celiac region is considered metastatic, and although resectable, the patient is considered to have stage IV disease.
Although operation is considered the “gold standard” in regard to staging, the goal of staging is to select patients who have disease that is potentially curable by resection. However, as mentioned, 80% of patients presenting with pancreatic cancer have advanced disease. Contemporary studies of patients with unresectable disease have suggested that median survival is limited, and an exploratory operation may be associated with significant perioperative morbidity, potential mortality, and diminished quality of life (25). In addition, the development of improved nonoperative techniques for relieving both biliary and gastric obstruction has reduced the need for operation. Thus, simply put, the goal of clinical staging is to accurately identify patients who would benefit from a curative resection while directing others toward more appropriate therapy.
Diagnostic Procedures: Cholangiography—Endoscopic Retrograde Cholangiography and Percutaneous Cholangiography
Cholangiography has long been an important component of the initial evaluation of the patient with painless jaundice. The earliest reports of endoscopic retrograde cholangiography (ERCP) claimed a sensitivity and specificity ranging from 46% to 94% and 94% to 100% for the diagnosis of pancreatic cancer (25,26,27). ERCP allows for imaging of the biliary and pancreatic ductal systems, and in 90% to 100% of patients with pancreatic cancer, it shows morphologic changes in the bile and/or pancreatic ducts such as irregularities, strictures, stenoses, and the dilatation of both ducts (“double duct” sign) (28,29,30,31) (Fig. 26.3). However, these changes can also be seen in chronic pancreatitis (32,33,34,35). The endoscopic and transhepatic approaches also allow for the collection of samples for cytologic confirmation of the cancer diagnosis. In addition, both approaches allow for the placement of temporary plastic stents to relieve biliary obstruction, and in unresectable patients, expanding metallic stents may be placed for long-term palliation. As a diagnostic modality, ERCP meets the criteria of high sensitivity and specificity with a minimally invasive risk profile (complication rates of 1%–2% after diagnostic ERCP and 5%–10% following therapeutic ERCP) (36,37,38,39). PTC is slightly more invasive (40); its role is less as a diagnostic tool and more for palliative therapy.
Since its introduction, computed tomography (CT) has replaced ERCP as the initial procedure of choice for diagnosis of pancreatic cancer (25,26,27), and the current primary role of ERCP is relieving malignant obstructive jaundice by placing plastic or expandable metallic stents (41,42). However, diagnostic ERCP could still be indicated in cases where the CT is normal but pancreatic cancer is suspected (43,44).
As staging modalities, ERCP and PTC do not address one of the main goals of staging, which is the determination of resectability.
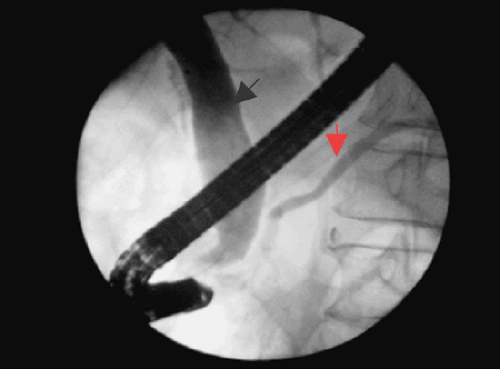 FIGURE 26.3. ERCP showing double duct sign—dilated pancreatic duct (red arrow) and dilated common bile duct (black arrow) (See also color Figure 26.3). |
Endoscopic Ultrasound
Conventional transabdominal ultrasound (US) has limited value in pancreatic cancer (45). Although transabdominal US is a noninvasive, safe, and relatively inexpensive imaging modality that visualizes liver lesions and biliary dilatation well, it is operator dependent, and its signal is attenuated by gas in the intestine precluding adequate imaging of the pancreatic parenchyma and the retroperitoneum. Recently, however, the positive and negative predictive values for resection of US are reported to be 89% and 79%, respectively, when lesions are visualized (46).
In 1980, the concept of endoscopic ultrasound (EUS) was introduced. It uses an echoendoscope, which incorporates a small high-frequency US transducer at its tip, permitting a high-resolution, real-time, B-mode scanning of the pancreas and surrounding structures when passed into the lumen of the gut. It allows the endoscopist to see within and beyond the wall of the luminal gastrointestinal tract. Three types of echoendoscopes are available: radial endoscope, which has a rotating US probe integrated into its tip with no possibility for needle aspiration; linear array endoscope, which obtain images along a plane parallel to the endoscope axis and with the capability of diagnostic (fine-needle aspiration cytology) and therapeutic interventions; and endoscopic probe, which is passed through the channel of a regular endoscope and provides high-resolution images parallel to the endoscope axis. In their randomized study, Gress et al. (47) showed that the radial and linear array echoendoscopes are equally accurate in staging pancreatic cancer. In 1984, Yasuda et al. (48) reported on their initial experience with the early prototype endoscopic transducers. They reported 10 patients who had CT scan, EUS, ERCP, and angiogram. EUS identified one lesion seen only on angiogram, one lesion seen by ERCP and CT only, and one final lesion not seen by the other modalities. This study defined the role of EUS in identifying small pancreatic lesions that are identified by symptoms in the periampullary region and not well visualized by other cross-sectional imaging.
In a prospective study, Rosch et al. (49) compared EUS, transabdominal US, CT, and ERCP in the diagnosis of 132 patients with suspected pancreatic cancer. Sensitivity and specificity were significantly higher for EUS (99% and 100%) than for US (67%/40%) and CT (77%/53%) and equal to ERCP (sensitivity 90%). They found that this difference was even more significant in small pancreatic cancers of ≤3 cm, and they concluded that in the evaluation of patients with suspected pancreatic tumors, EUS should be considered early in the staging process.
A comparative study of Legmann et al. (80) of 32 patients with suspected pancreatic tumor comparing dual-phase helical CT and EUS for the diagnosis and staging of pancreatic tumors showed overall diagnostic sensitivity of 92% for dual-phase helical CT and 100% for EUS. Both dual-phase helical CT and EUS had overall accuracy for staging of pancreatic tumors of 93% and overall accuracy for predicting resectability of 90%; the accuracy of predicting unresectability was 100% and 86% for dual-phase helical CT and EUS, respectively (p >0.80). Other studies (50,51,66) have also shown that EUS is inaccurate in assessing superior mesenteric artery tumor involvement. This is because of the distance of superior mesenteric artery from the endoscopic probe in the lumen of the duodenum, and this obviously affects the prediction for unresectability. In the assessment of major venous invasion, spiral CT and gadolinium-enhanced MRI showed similar results as EUS (79). In a study of 62 patients with pancreatic cancer comparing EUS, helical CT, MRI, and angiography in the preoperative staging and tumor resectability assessment of pancreatic cancer, Soriano et al. (75) showed by using univariate logistic regression analysis that helical CT had the highest accuracy in assessing extent of primary tumor locoregional extension, vascular invasion, distant metastases, tumor TNM stage, and tumor resectability, but in assessing tumor size and lymph node involvement, EUS had the highest accuracy. Very small lymph nodes <5 mm can be detected by EUS; however, nodal tumor involvement staging accuracy by EUS, although better than CT or MRI, has been shown to range from 64% to 82% (52). This is because of its inability to distinguish malignant infiltration from benign inflammation of the lymph nodes. Nodal staging of pancreatic tumors by EUS, however, has been enhanced by the addition of EUS-guided fine-needle aspiration, which allows for a tissue diagnosis of the primary tumor as well as lymph nodes, liver metastasis, and peritoneal/pleural fluid, useful in the context of unresectable disease or neoadjuvant therapy (53). Studies have shown it to have sensitivity and specificity rates of 75% to 90% and 94% to 100%, and a complication rate of 1% (54,55,56). These studies underscore the value of EUS in identifying small lesions and determining the status of peripancreatic vessels in patients with complex pancreatic pathology.
Another important role of EUS is its therapeutic capabilities, and as Fazel and Draganov (57) pointed out, EUS is evolving rapidly from a primarily diagnostic role to one of therapeutic intervention. Therapeutic interventions of EUS include celiac neurolysis and nerve block, fine-needle tumor injection with antitumor agents such as activated lymphocytes and viral gene vectors, delivery of photodynamic therapy, and transmural pseudocyst drainage (57,58).
Computed Tomography
CT was introduced into clinical practice in 1975 and subsequent refinements in CT technology have radically changed the staging of pancreatic cancer. It has become the standard staging modality for pancreatic tumors (59). CT can identify patients with significant metastatic disease without exploratory laparotomy. CT-guided needle biopsy allowed tissue confirmation in these advanced patients. The routine use of CT scanning in the staging of patients with pancreatic tumors has led to improved patient selection. CT yielded sensitivities in the range 66% to 97% and specificities of 53% to 69% in the studies of conventional dynamic CT in the diagnosis of pancreatic malignancy conducted in the 1980s and early 1990s (60,61,62,63,64,65,66,67,68). CT scanning allows accurate identification of hepatic metastases over 1 cm in diameter (69).
Although any lesion visualized outside the pancreas makes staging simple, the difficulty arises in the absence of visible metastatic disease. The determination of resectability by CT is based on a clear fat plane around the splenic, mesenteric artery and the celiac and hepatic arteries (Fig. 26.4).
Selections of recent studies (68,69,72,73,74,75,76,77,80,81,82,84) evaluating the utility of CT scanning in the determination of resectability by these criteria are displayed in Table 26.2. Studies by Freeny et al. (68) and McCarthy et al. (77) evaluated axial CT scanning coordinated with intravenous contrast injection (dynamic), and found adequate identification of the primary tumor and hepatic parenchymal lesions. However, there was reduced accuracy in assessment of portal nodes, portal vein involvement, extension into the root of the mesentery, and posterior extension of tumor. The introduction of spiral (helical) CT scanning with multiple detectors and dual-phase contrast enhancement have improved our ability to image the portal vein and the visceral arteries in relation to the primary tumor (82), a critical component of the determination of resectability. Spiral CT images a volume of tissue while the patient moves through the scanner tube, usually during a single breathhold (65). Dual-phase CT scanning takes advantage of the short
acquisition time of the spiral CT scanner and images the pancreas first during its maximum enhancement and then rescans through the liver during the portal phase to obtain maximum enhancement of liver lesions. Subsequent reports with this technique have improved visualization of local tumor extension as well (69,80,81,82,83). In these studies, as in practice, prediction of resectability remains somewhat subjective, resulting in a number of reports with low positive predictive value of CT scanning for the identification of resectability. Saldinger et al. (84) defined unresectable to include only definitive evidence of metastasis or vascular invasion. Using these criteria when interpreting spiral CT angiography, they were able to increase the sensitivity and negative predictive value for resection to 100%. However, the specificity and positive predictive value remained disappointingly low at 39% to 86% and 50% to 89%, respectively, as also reported by other authors (60,66). Spiral CT angiogram uses a three-dimensional data set from spiral scan to reconstruct the peripancreatic vessels. In addition, this data set can be displayed as an angiogram, allowing the surgeon to evaluate the important vessels without a conventional angiogram (79). These studies evaluated all patients with pancreatic tumors; the stage distribution was weighted toward advanced disease. This makes detection of metastatic disease or obvious signs of locally advanced disease more likely. However, spiral CT has been shown to miss small liver lesions and borderline lymphadenopathy identified at laparotomy or laparoscopy with studies reporting sensitivities of approximately 75% in the detection of small liver and/or peritoneal metastases (82), (83,85,86). Although approximately 25% to 30% of patients with radiologically resectable disease will ultimately be unresectable at surgery (61,68,87), CT scanning has nonetheless become the standard method for diagnosing and staging pancreatic cancer (54).
acquisition time of the spiral CT scanner and images the pancreas first during its maximum enhancement and then rescans through the liver during the portal phase to obtain maximum enhancement of liver lesions. Subsequent reports with this technique have improved visualization of local tumor extension as well (69,80,81,82,83). In these studies, as in practice, prediction of resectability remains somewhat subjective, resulting in a number of reports with low positive predictive value of CT scanning for the identification of resectability. Saldinger et al. (84) defined unresectable to include only definitive evidence of metastasis or vascular invasion. Using these criteria when interpreting spiral CT angiography, they were able to increase the sensitivity and negative predictive value for resection to 100%. However, the specificity and positive predictive value remained disappointingly low at 39% to 86% and 50% to 89%, respectively, as also reported by other authors (60,66). Spiral CT angiogram uses a three-dimensional data set from spiral scan to reconstruct the peripancreatic vessels. In addition, this data set can be displayed as an angiogram, allowing the surgeon to evaluate the important vessels without a conventional angiogram (79). These studies evaluated all patients with pancreatic tumors; the stage distribution was weighted toward advanced disease. This makes detection of metastatic disease or obvious signs of locally advanced disease more likely. However, spiral CT has been shown to miss small liver lesions and borderline lymphadenopathy identified at laparotomy or laparoscopy with studies reporting sensitivities of approximately 75% in the detection of small liver and/or peritoneal metastases (82), (83,85,86). Although approximately 25% to 30% of patients with radiologically resectable disease will ultimately be unresectable at surgery (61,68,87), CT scanning has nonetheless become the standard method for diagnosing and staging pancreatic cancer (54).
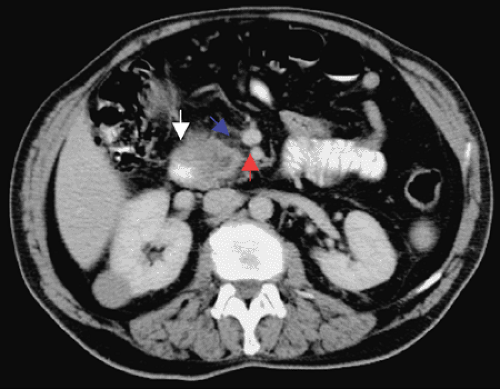 FIGURE 26.4. CT showing clear fat pad between head of pancreas tumor (white arrow) and the superior mesenteric vessels (blue arrow, superior mesenteric vein; red arrow, superior mesenteric artery) (See also color Figure 26.4). |
Table 26.2 Measures of Accuracy of computed tomography Scanning for the Prediction of Resectability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree