J. Curtis Nickel, MD, FRCSC
Prostatitis and Related Conditions
Historical Aspects
Modern descriptions of the clinical presentation, pathology, and microscopic evaluation of prostate-specific specimens of prostatitis patients were firmly established by Young, Gereghty, and Stevens (1906) by the turn of the 20th century. Bacterial and cytologic localization studies of the lower urinary tract were described shortly thereafter (Hitchens and Brown, 1913) and standardized by 1930 (Von Lackum, 1927, 1928; Nickel, 1930, 1999a). The primary form of therapy for prostatitis during most of the 20th century was repetitive prostate massage (Farman, 1930; O’Conor, 1936; Henline, 1943; Campbell, 1957). With the introduction of sulfanilamide in the 1930s, antimicrobial therapy became the main therapeutic approach (Ritter and Lippow, 1938). However, even in the 1950s and 1960s, the significance of inflammatory cells and bacteria in the expressed prostatic secretion (EPS) was questioned (O’Shaughnessy et al, 1956; Bowers and Thomas, 1958; Bourne and Frishette, 1967), and it was even recognized that, in many cases, antibiotics were performing little better than placebo in the treatment of prostatitis (Gonder, 1963).
The next era of prostatitis management began in the 1960s with Meares and Stamey’s (1968) description of the four-glass lower urinary tract segmented localization study. Prostatic massage as the mainstay of prostatitis therapy was abandoned, and antimicrobial therapy was rationalized for the very small percentage of patients with bacteria localized to prostate-specific specimens. Unfortunately the vast majority of patients who were diagnosed with a nonbacterial cause continued to suffer the indignities of dismal urologic management (Nickel, 1998a). The establishment of new definitions and a classification system, better understanding of the etiopathogenesis, completion of randomized placebo-controlled trials with validated outcome indices, and the evolving insight that patients with prostatitis have variable clinical phenotypes has radically changed the way this condition is managed.
Epidemiology
Prostatitis is the most common urologic diagnosis in men younger than age 50 years and the third most common urologic diagnosis in men older than age 50 years after benign prostatic hyperplasia (BPH) and prostate cancer (McNaughton Collins et al, 1998). There were almost 2 million U.S. physician visits annually from 1990 to 1994 with prostatitis listed as the diagnosis (McNaughton Collins et al, 1998), Approximately 5% of visits to U.S. urologists were reported to be for inflammatory diseases of the prostate (Schappert, 1994). In the mid 1990s urologists in Wisconsin in the United States saw an average of 173 patients with prostatitis per year (Moon, 1997) whereas Canadian urologists reported an average of 264 patients with prostatitis per year, with 38% of whom newly diagnosed (Nickel et al, 1998a). Based on both a physician survey study in Dane County, Wisconsin (Moon, 1997), and a survey of younger men from a Wisconsin National Guard unit (Moon et al, 1997), it was estimated that 5% of men (aged 20 to 50 years) have a history of prostatitis. In the Netherlands, de la Rosette and colleagues (1992a) noted that 4% of respondents reported a history of prostatitis, whereas researchers in Finland reported a 14% overall lifetime prevalence in that country (Mehik et al, 2000). McNaughton Collins and colleagues (2002) noted that 16% of health care professionals in the United States report either a previous or current diagnosis of prostatitis. Roberts and associates (1998) reviewed the medical records of 2113 men from July 1992 through February 1996 (a median of 50 months’ follow-up) in the Olmsted County community-based cohort in Minnesota and found the overall prevalence of a physician’s diagnosis of prostatitis was 9%.
Many of these epidemiologic studies are limited by physicians’ or patients’ long-term recollection and the unreliability of a physician’s coding or diagnosis of prostatitis. Population-based studies employing the validated National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) (Litwin et al, 1999) to determine the prevalence of prostatitis-like symptoms in the general population of men showed widely variant results (12.2% in Nigeria [Ejike and Ezeanyika, 2008], 8.0% in Malaysia [Cheah et al, 2003], 6.6% in Canada [Nickel et al, 2001b], 2.7% in Singapore [Tan et al, 2002], and approximately 2.2% in older men in Olmsted County [Roberts et al, 2002]). Employing NIH-CPSI definitions of prostatitis-like symptoms in a managed care population, Clemens and colleagues (2006) estimated that between 5.9% and 11.2% have prostatitis-like symptoms depending on the specific definition. Whereas retrospective billing data studies have indicated that up to 8% of U.S. male patients seen in urologic outpatient practice have a diagnosis of prostatitis (McNaughton Collins et al, 1998), prospective practice audit studies have shown that as many as 12% of men presenting to Italian urologists are diagnosed with prostatitis (Rizzo et al, 2003), whereas only 2.8% of men had a clinical diagnosis of prostatitis in a similar study carried out in Canada (Nickel et al, 2005c). Symptoms of prostatitis wax and wane, with approximately one third to one half of patients experiencing relief of symptoms over a 1-year period (Nickel et al, 2002b; Turner et al, 2004b, Propert et al, 2006b).
It has traditionally been believed that prostatitis is a disease of younger men (e.g., aged 35 to 50 years). Epidemiologic studies described in this section confirm that prostatitis affects men of all ages, unlike BPH and prostate cancer, which are predominantly diseases of older men. Compared with men aged 66 years and older, a diagnosis of prostatitis is 1.6-, 2.6-, and 2.1-fold greater in men aged 18 to 35, 36 to 50, and 51 to 65 years, respectively, in the Olmsted County study (Roberts et al, 1998). The age-specific prevalence of a physician-assigned diagnosis of prostatitis was highest in patients between the ages of 20 and 49 years and increased again in those older than 70 years. The cumulative probability of having a diagnosis of prostatitis (acute or chronic) by 85 years of age was 26%. In the Canadian prevalence study (Nickel et al, 2001b) there was no significant difference in the prevalence of prostatitis-like symptoms in men younger than 50 years (11.5% reported at least mild symptoms over the previous week) compared with men older than 50 years (8.5% reported at least mild symptoms over the previous week). As many as 20% of men diagnosed with BPH also report prostatitis-like symptoms (Nickel 2005a). Interestingly, although prostatitis symptoms appear to be as prevalent in older versus younger males they are reported to be less distressing with age (Hedelin and Jonsson, 2007).
Chronic prostatitis is associated with substantial costs and significant predicted resource consumption (Calhoun et al, 2004; Turner et al, 2004a; Duloy et al, 2007). Overall spending in the United States for the diagnosis and management of prostatitis, exclusive of pharmaceutical spending, totaled 84 million dollars in 2000 and appears to be increasing (Pontari et al, 2007). This economic factor needs increased attention when evaluating the incidence and treatment of this prevalent condition.
Histopathology
For the pathologist, prostatitis is defined as an increased number of inflammatory cells within the prostatic parenchyma (Cotran et al, 1999). Prostatic inflammation may or may not be noted in patients with a diagnosis of prostatitis (True et al, 1999), BPH (Nickel et al, 1999c), or prostate cancer (Zhang et al, 2000) and is noted in autopsy series in as many as 44% of prostate tissue samples from men without any definitive prostate disease (McNeal, 1968).
Key Points
Epidemiology
A consistent description of fairly distinct, although often coexisting, patterns of chronic inflammation can be found in the prostate gland of patients with or without prostate disease. The most common pattern of inflammation is a lymphocytic infiltrate in the stroma immediately adjacent to the prostatic acini (Kohnen and Drach, 1979; Nickel et al, 1999b). The intensity of the inflammatory process varies considerably from only scattered lymphocytes to dense lymphoid nodules. Stromal lymphocytic infiltrates frequently coexist with periglandular inflammation. Sheets, clusters, and occasional nodules of lymphocytes and scattered plasma cells are seen within the fibromuscular stroma with no apparent relationship to the ducts and acini. Infiltrates of inflammatory cells restricted to the glandular epithelium and lumen are found in association with prostatitis and BPH but can be found in asymptomatic patients. The intraepithelial inflammatory cells may be neutrophils, lymphocytes, or macrophages, or all of these, whereas neutrophils and macrophages are typically found in the lumen. Figure 11–1 illustrates the various inflammatory patterns seen in a prostate specimen of a patient with chronic prostatitis.
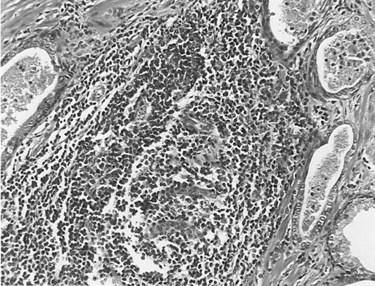
(Courtesy of Dr. Alexander Boag.)
Corpora amylacea, which may develop from the deposition of prostatic secretions around a sloughed epithelial cell or other irritant, are not usually associated with inflammation unless they become large enough to distend or obstruct the prostatic gland (Attah, 1975). Prostatic calculi may contribute to prostatic inflammation by obstructing central prostate ducts and thus preventing drainage or providing a nidus in which bacteria can survive host defenses and antibiotics (Meares, 1974; Roberts et al, 1997).
Granulomatous prostatitis presents a nonspecific and variable histologic pattern typified by heavy lobular, mixed, inflammatory infiltrates that include abundant histiocytes, lymphocytes, and plasma cells. Small, discrete granulomas may be present, or the pattern may be typified by well-defined granulomas. Granulomatous prostatic inflammation is a common consequence of surgery (Eyre et al, 1986) or therapy with bacillus Calmette-Guérin (BCG) (Lafontaine et al, 1997) and a rare event in patients with systemic tuberculosis (Saw et al, 1993).
A consensus group of urologists and pathologists have developed a classification system to describe histologic inflammatory patterns in the prostate (Nickel et al, 2001d), but it is only useful for comparative research purposes.
Etiology
Microbiologic
Gram-Negative Uropathogens
Acute bacterial prostatitis is a generalized infection of the prostate gland and is associated with both lower urinary tract infection (UTI) and generalized sepsis. Chronic bacterial prostatitis is associated with recurrent lower UTIs (i.e., cystitis) secondary to areas of focal uropathogenic bacteria residing in the prostate gland. The most common cause of bacterial prostatitis is the Enterobacteriaceae family of gram-negative bacteria, which originate in the gastrointestinal flora. The most common organisms are strains of Escherichia coli, which are identified in 65% to 80% of infections (Stamey, 1980; Lopez-Plaza and Bostwick, 1990; Weidner et al, 1991b; Schneider et al, 2003). Pseudomonas aeruginosa, Serratia species, Klebsiella species, and Enterobacter aerogenes are identified in a further 10% to 15% (Meares, 1987; Weidner et al, 1991b). However, in acute bacterial prostatitis, the organisms that result from previous manipulation of the lower urinary tract show different patterns of virulence and resistance (e.g., to ciprofloxacin and cephalosporins) compared with the organisms associated with spontaneous acute prostatitis (Millan-Rodriquez et al, 2006; Ha et al, 2008).
Urovirulence factors play a significant role in the pathogenesis of bacterial prostatitis (Ruiz et al, 2002; Johnson et al, 2005). For instance, bacterial P-fimbria (or pili) bind to the urothelial receptors, and this subsequently facilitates ascent into the urinary tract as well as establishing deep infections in the prostate gland itself (Dilworth et al, 1990; Neal et al, 1990; Andreu et al, 1997). Colonization of the lower urinary tract by E. coli is also facilitated by the presence of the type 1 fimbria, also known as mannose-sensitive fimbria. The receptor is a common moiety of the uroepithelial uromucoid; this association has been shown to be important in the development of cystitis in humans, and its presence in prostatitis has also been documented (Correll et al, 1996). Phase variation of type 1 pili during the establishment of acute bacterial prostatitis may occur in the setting of prostatitis (Schaeffer, 1991). Multiple virulence factors appear to be necessary to produce prostatitis (Mitsumori et al, 1999; Ruiz et al, 2002). Bacteria reside deep in the ducts of the prostate gland and when threatened, with host defense and antimicrobial therapy, tend to form aggregates (also called biofilms), which appears to be a protective mechanism allowing bacteria to persist in the prostate gland even when the cystitis is treated with antibiotics (Nickel and Costerton, 1993; Nickel et al, 1994). Hemolysin appears to be a virulence factor associated with E. coli acute prostatitis, but hemolysin may also be associated with increased ability of certain strains of E. coli to persist in the prostate as biofilms in patients with chronic bacterial prostatitis (Soto et al, 2007).
Gram-Positive Bacteria
Enterococci are believed to account for 5% to 10% of documented prostate infections (Drach, 1974a; Meares, 1987; Bergman, 1994). The role of other gram-positive organisms, which are also commensal organisms in the anterior urethra, is controversial (Jimenez-Cruz et al, 1984; Fowler and Mariano, 1984b, Krieger et al, 2002). An etiologic role for gram-positive organisms such as Staphylococcus saprophyticus, hemolytic streptococci, Staphylococcus aureus, and other coagulase-negative staphylococci has been suggested by a number of authors (Drach, 1974a, 1986; Bergman, 1994). Nickel and Costerton (1992) have shown coagulase-negative Staphylococcus to be present in the EPS as well as transperineal prostate biopsy tissue of men with chronic prostatitis (microscopy and culture). Although this and other studies (Carson et al, 1982; Pfau, 1983; Bergman et al, 1989; Wedren, 1989) suggested that coagulase-negative staphylococci are involved in the pathogenesis of chronic prostatitis, these studies did not conclusively demonstrate that these bacteria were actually causing the inflammation and symptom-complex rather than simply colonizing the prostate (Krieger et al, 2002). However, eradication of gram-positive bacteria in the prostate of men experiencing recent onset of prostatitis symptoms resulted in similar clinical results compared with men with gram-negative uropathogens localizing to the prostate (Magri et al, 2007a; Nickel and Xiang, 2008). In both cases, eradication of the bacteria localized to the prostate was strongly correlated with a good clinical outcome. However, the inconsistent localization of gram-positive bacteria in prostate-specific specimens from patients with chronic prostatitis suggests that this relationship may not be as strong as suggested (e.g., Krieger et al, 2005).
Anaerobic Bacteria
In studies in which the prostate-specific specimens were cultured anaerobically, anaerobic bacteria could be identified in a small number of patients (Nielsen and Justesen, 1974; Mardh and Colleen, 1975; Szoke et al, 1998). This has not been a consistent finding, and the role of anaerobic bacteria is essentially unknown.
Corynebacterium Infection
Corynebacterium species have usually been acknowledged as prostate nonpathogens but have been suggested as potential etiologic agents in this disease (Riegel et al, 1995; Domingue, 1998). Domingue and colleagues (1997) suggested that these difficult-to-culture coryneforms could be missed by routine culturing of EPS. Direct Gram staining of the EPS showed gram-variable pleomorphic coccobacillary rods that do not usually grow on routine media. The presence of these pleomorphic swollen rods was also shown by fluorescent acridine orange staining. Tanner and associates (1999), using polymerase chain reaction (PCR) techniques, were able to identify a bacterial signal (phylogenetically gram-positive organisms with predominance of Corynebacterium species) in 65% of 17 patients with chronic prostatitis. Approximately half of these patients tended to respond to antimicrobial therapy, whereas patients in whom molecular signals for these bacteria could not be identified did not.
Chlamydial Infection
The evidence supporting the role of Chlamydia trachomatis as an etiologic agent in chronic prostatic inflammation is both confusing and conflicting. Mardh and Colleen (1972) found that one third of men with chronic prostatitis had antibodies to C. trachomatis compared with 3% of controls. Shortliffe and coworkers (1992) found that 20% of patients with nonbacterial prostatitis had antichlamydial antibody titers in the prostatic fluid. Koroku and associates (1995) detected C. trachomatis–specific immunoglobulin A (IgA) in 29% of men with chronic nonbacterial prostatitis. Bruce and colleagues (1981) found that 56% of patients with “subacute or chronic prostatitis” were infected with C. trachomatis (after examining early morning urine, prostatic fluid, or semen specimens). In a follow-up study, Bruce and Reid (1989) found that 6 of 55 men with abacterial prostatitis, including 31 believed to have chlamydial prostatitis, met strict criteria for positive diagnosis for chlamydial prostatitis based on identification of the organisms by culturing or immunofluorescence. Kuroda and colleagues (1989) identified C. trachomatis in the urethras of 20% of men with prostatitis. Other investigators have come to similar conclusions (Nilsson et al, 1981; Weidner et al, 1983). Chlamydia has also been isolated in prostate tissue specimens. Poletti and coworkers (1985) isolated C. trachomatis from prostate samples obtained by transrectal aspiration biopsy of men with “nonacute abacterial prostatitis.” Abdelatif and colleagues (1991) identified intracellular Chlamydia employing “in-situ hybridization techniques” in transurethral prostate chips from 30% of men with histologic evidence of “chronic abacterial prostatitis.” Shurbaji and associates (1998) identified C. trachomatis in paraffin-embedded secretions in 31% of men with histologic evidence of prostatitis compared with none in patients with BPH without inflammation.
Although Mardh and Colleen (1972) suggested that C. trachomatis may be implicated in as many as one third of men with chronic prostatitis, their follow-up studies employing culturing and serologic tests could not confirm C. trachomatis as an etiologic agent in idiopathic prostatitis (Mardh and Colleen, 1975; Mardh et al, 1978). Shortliffe and Wehner (1986) came to a similar conclusion when her group evaluated antichlamydial antibody titers in prostatic fluid. Twelve percent of controls (compared with 20% of patients with nonbacterial prostatitis) had detectable antibodies. Berger and coworkers (1989) could not culture C. trachomatis from the urethras in men with chronic prostatitis nor did they find a serologic or local immune response to C. trachomatis in such patients. Doble and associates (1989b) were not able to culture or detect by immunofluorescence Chlamydia in transperineal biopsy specimens of abnormal areas of the prostate in men with chronic abacterial prostatitis. Krieger and colleagues (1996b) were only able to find Chlamydia in 1% of prostate tissue biopsy specimens in men with chronic prostatitis. A further localization and culture series by Krieger and associates (2000) also failed to culture Chlamydia from either urethral or prostate specimens. Further elucidation of the role of chlamydial etiology of prostate infection is required to make any definitive statement on the association between isolation of this organism and its prostatic origin and effect (Weidner et al, 2002).
Ureaplasma Infection
Ureaplasma urealyticum is a common organism isolated from the urethra of both asymptomatic men and men with nonspecific urethritis. Weidner and colleagues (1980) found high U. urealyticum concentrations in prostate-specific specimens in patients with signs and symptoms of abacterial prostatitis. Isaacs (1993) cultured U. urealyticum from prostate secretion in 8% of patients with chronic nonbacterial prostatitis. Fish and Danziger (1993) found significant U. urealyticum concentrations in 13% of patients with prostatitis. Treatment with specific antimicrobial therapy cleared the organisms in all cases. Ohkawa and associates (1993a) isolated U. urealyticum cells from the prostates of 18 of 143 patients with chronic prostatitis. Antibiotics eradicated the organism in all, improved the symptoms in 10, and cleared the leukocytes in the EPS in 4 (Ohkawa et al, 1993b).
Other investigators (Mardh and Colleen, 1975), employing similar techniques, were unable to implicate U. urealyticum in patients with nonbacterial prostatitis. The problems encountered in all these studies include the absence of controls and the fact that it was difficult to account for possible urethral contamination in collecting specific prostate specimens.
Other Microorganisms
Candida (Golz and Mendling, 1991; Indudhara et al, 1992) and other mycotic infections such as aspergillosis and coccidioidomycosis (Schwarz, 1982; Chen and Schijj, 1985; Campbell et al, 1992; Truett and Crum, 2004) have been implicated in prostatic inflammation. However, in most cases it was usually an isolated finding in immunosuppressed patients or those with systemic fungal infection. Viruses (Doble et al, 1991; Benson and Smith, 1992) have also been implicated in prostatic inflammation, but no systematic evaluation of the role of these agents in prostatitis has been undertaken. Trichomonas has been described in the prostate glands of patients complaining of prostatitis-like symptoms (Kuberski, 1980; Gardner et al, 1996; Skerk et al, 2002a).
Nonculturable Microorganisms
There are significant limitations to the culture techniques employed to attempt to identify etiologic microorganisms associated with prostatitis (Lowentritt et al, 1995; Domingue et al, 1997; Domingue, 1998). Bacteria may exist in aggregated biofilms adherent to the prostatic ductal walls or within the obstructed ducts in the prostate (Nickel and MacLean, 1998). Nickel and Costerton (1993) observed that 60% of patients with previously diagnosed chronic bacterial prostatitis who progressed to sterile EPS cultures but continued to have symptoms despite antimicrobial therapy had positive cultures in prostate biopsy specimens showing an organism similar to the initial organism. As discussed earlier, such organisms appear to persist in small aggregates or biofilms in the ducts and acini of the prostate gland.
Berger and associates (1997) cultured urine specimens and transperineal prostate biopsy specimens specifically for commensal and fastidious organisms. These investigators demonstrated that, in prostate biopsy cultures, men with evidence of inflammation in EPS are more likely to have bacteria isolated, positive cultures for anaerobic bacteria, higher total bacterial counts, and more bacterial species isolated than men without EPS inflammation. Krieger and colleagues (1996b), Riley and coworkers (1998), and Tanner and associates (1999) used a combination of clinical, culture, and molecular biologic methods (i.e., PCR) and found a strong correlation between inflammation and EPS and the detection of bacteria-specific 16S rRNA (gram-negative and gram-positive organisms) in the prostate tissue. But other researchers did not show any association between culture and PCR findings in men with nonbacterial prostatitis compared with men with prostatitis symptoms (Keay et al, 1999; Lee et al, 2003; Leskinen et al, 2003b). Nanobacteria are intriguing organisms that are difficult to isolate and culture but may be implicated in some chronic urologic conditions, including chronic prostatitis (Wood and Shoskes, 2006). A number of investigators (Shoskes et al, 2005; Short et al, 2008) have demonstrated the possibility that nanobacteria associated with and without prostatic calculi may be implicated in some cases of chronic prostatitis.
It has been estimated that less than 10% of all environmental bacteria have been identified (Domingue, 1998), so it is possible that fastidious and nonculturable microorganisms might be present in the prostate gland and that such organisms might be involved in the inflammatory process and subsequent development of symptoms.
Altered Prostatic Host Defense
Risk factors that allow bacterial colonization or infection of the prostate with potentially pathogenic bacteria include intraprostatic ductal reflux (Kirby et al, 1982); phimosis (VanHowe, 1998); specific blood groups (Lomberg et al, 1986); unprotected penetrative anal rectal intercourse; UTI; acute epididymitis (Berger et al, 1987); indwelling urethral catheters and condom catheter drainage (Meares, 1998); and transurethral surgery, especially in men who have untreated, infected urine (Meares, 1989). Secretory dysfunction of the prostate characterized by an alteration in the composition of prostatic secretions can be diagnostic of patients with prostatitis: there is a decrease in the levels of fructose; citric acid; acid phosphatase; the cations zinc, magnesium, and calcium; and the zinc-containing prostatic antibacterial factor; but pH, the ratio of isoenzymes lactate dehydrogenase-5 to lactate dehydrogenase-1, levels of inflammatory proteins such as ceruloplasmin, and complement C3 are increased (Meares, 1989). These defined alterations in the prostate secretory function have also been blamed for adversely affecting the normal antibacterial nature of prostatic secretions. A decrease in prostatic antibacterial factor may reduce the intrinsic antibacterial activity of the prostatic fluid (Fair et al, 1976), whereas the alkaline pH may hamper diffusion of certain basic antimicrobial drugs into the prostatic tissue and fluid (Fair and Cordonnier, 1978). However, caution is warranted because it is not known whether these compositional changes are a cause or a consequence of inflammation.
Dysfunctional Voiding
Anatomic or neurophysiologic obstruction resulting in high-pressure dysfunctional flow patterns has been implicated in the pathogenesis of the prostatitis syndrome. Blacklock (1974, 1991) demonstrated that bladder neck, prostatic, and urethral anatomic abnormalities predisposed some men to developing prostatitis. Urodynamic studies confirm that many patients, particularly those with prostatodynia, have decreased maximal urinary flow rates and obstructive-appearing flow patterns (Barbalias et al, 1983; Ghobish, 2002). On video-urodynamic studies, many patients with prostatitis syndromes show incomplete funneling of the bladder neck as well as vesicourethral dyssynergic patterns (Kaplan et al, 1994, 1997; Hruz et al, 2003). Investigators (Dellabella et al, 2006) have described ultrasound alterations of the preprostatic sphincter in men with chronic prostatitis. In a study of 48 treatment refractory chronic prostatitis patients with no associated infection, Hruz and associates (2003) determined that 29 (60%) had bladder neck hypertrophy diagnosed by endoscopic and urodynamic criteria. This dyssynergic voiding may lead to an autonomic overstimulation of the perineal-pelvic neural system with subsequent development of a chronic neuropathic pain state. Alternatively, this high-pressure, dysfunctional voiding may result in intraprostatic ductal reflux in susceptible individuals (see the next section).
Intraprostatic Ductal Reflux
Reflux of urine and possibly bacteria into the prostatic ducts has been postulated as one of the most important etiologic mechanisms involved in the pathogenesis of chronic bacterial and nonbacterial prostatic inflammation. Anatomically, the ductal drainage of the peripheral zone is more susceptible than other prostatic zones to intraprostatic ductal reflux (Blacklock, 1974, 1991). Kirby and associates (1982) instilled a carbon particle solution into the bladders of men diagnosed with nonbacterial prostatitis. Carbon particles were found in the EPS macrophages and prostatic acini and ductal system after surgery in men with nonbacterial prostatitis. Persson and Ronquist (1996) noted high levels of urate and creatinine in EPS, which they postulated was caused by urine reflux into the prostatic ducts. Terai and coworkers (2000) provided molecular epidemiologic evidence for ascending infection in acute bacterial prostatitis.
Prostatic calculi are composed of substances found only in urine, not in prostatic secretions (Sutor and Wooley, 1974; Ramiraz et al, 1980), further evidence that urinary intraprostatic reflux occurs and likely contributes to the formation of prostatic calculi. If pathogenic bacteria reflux into the prostate gland, they may exist in protected aggregates within prostatic calculi themselves. High culture counts of pathogens encrusted in prostatic calculi have been demonstrated by Eykyn and colleagues (1974). This type of bacterial colonization in protective bacterial aggregates or biofilms associated with prostatic calculi may lead to recalcitrant chronic prostatitis and subsequent recurrent UTIs despite what seems to be adequate antibiotic therapy. Ludwig and coworkers (1994), employing transrectal ultrasonography, showed that men with chronic inflammatory prostatitis had a significantly increased frequency of prostatic calculi compared with men without prostate inflammation (prostatodynia). It appears that prostatic calcification is common in patients with nonbacterial chronic prostatitis and is associated with greater inflammation, bacterial colonization, pelvic floor spasm, and symptom duration (Shoskes et al, 2007). The inflammation resulting from chemical, bacterial, or immunologic stimulation has been shown to possibly cause an increase in intraprostatic pressures, measurable with transperineally inserted pressure transducers (Mehik et al, 2002).
Immunologic Alterations
The local prostatic immune system is activated by infection in bacterial prostatitis. In acute bacterial prostatitis, serum and prostatic fluid antigen-specific (i.e., bacterial antigen) IgG and IgA can be detected immediately after the onset of infection, and after successful antibiotic therapy they decline to normal levels over the next 6 to 12 months (Fowler and Mariano, 1984a; Meares, 1977, 1998; Kumon, 1992). Prostate-specific antigen (PSA) levels can be markedly elevated during an acute episode of bacterial prostatitis (Dalton, 1989; Moon et al, 1992; Neal et al, 1992) and slowly resolve to normal levels over the course of 6 weeks, provided there is no recrudescence of the infection. In chronic bacterial prostatitis, no serum immunoglobulin elevation is detected, whereas prostatic fluid IgA and IgG levels are both increased (Shortliffe and Wehner, 1986; Kumon, 1992). After successful antibiotic therapy, IgG levels return to normal after several months but the IgA (particularly secretory IgA) levels remain elevated for almost 2 years (Shortliffe et al, 1981a, 1981b; Fowler and Mariano, 1984a). Antibody-coated bacteria detected in urine, EPS, and semen is another prominent feature of chronic bacterial prostatitis (Riedasch et al, 1984, 1991).
Noninfectious inflammation (nonbacterial prostatitis) might also be secondary to immunologically mediated inflammation due to some unknown antigen or perhaps even related to an autoimmune process. IgA and IgM antibody levels (not microorganism-specific) are elevated (Shortliffe and Wehner, 1986; Shortliffe et al, 1989, 1992), and similar antibodies as well as fibrinogen and complement C3 (Vinje et al, 1983; Doble et al, 1990) have been identified in prostatic biopsy specimens from patients with chronic prostatitis. Both animal model studies (Donadio et al, 1998; Ceri et al, 1999; Lang et al, 2000) and human studies (Alexander et al, 1997; Batstone et al, 2002; Maake et al, 2003; Motrich et al, 2007) have suggested that prostatitis may be an autoimmune process. A number of candidates have been suggested for the self-antigen, including PSA (Ponniah et al, 2000). Other specific immunologic and neuroendocrine alterations such as cytokine production (Alexander et al, 1998; Jang et al, 2003) and nerve growth factor (Miller et al, 2002) have a subsequent role to play in the process of inflammation. Specifically, interleukin-10 has been implicated in the etiology and clinical manifestations in chronic prostatitis (Miller et al, 2002; Shoskes et al, 2002) but other cytokines such as interleukin (IL)-1β and tumor necrosis factor-α have also been implicated (Nadler et al, 2000). IL-8 is the most common cytokine localized to the semen in men with chronic prostatitis (Khadra et al, 2006; Penna et al, 2007). There may be a genetic phenotype that promotes specific immunologic parameters that predispose to immunologically induced prostatic inflammation (Riley et al, 2002; Shoskes et al, 2002). These immunophenotypic patterns have even been observed in noninflammatory category IIIB chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) (Barghorn et al, 2001). Whatever the initiating event, the immunologic cascade appears to have an important role in the development of prostatitis in those patients who develop prostatic inflammation (Moon, 1998; Kumon, 1999).
Chemically Induced Inflammation
Investigators have demonstrated that urine and its metabolites (e.g., urate) are present in the prostatic secretion of patients with chronic prostatitis (Persson and Ronquist, 1996). These investigators have hypothesized that the prostatic inflammation and subsequent symptoms may be simply due to a chemically induced inflammation secondary to the noxious substances in the urine that have refluxed into the prostatic duct.
Pelvic Floor Muscle Abnormalities
Some investigators (Zermann et al, 1999) propose that the sensory or motor disturbances or both consistent with neural dysregulation of the lower urinary tract may be a consequence of acquired abnormalities in the central nervous system. Certainly, extraprostatic tenderness is identified in many patients with chronic prostatitis (Berger et al, 2007; Shoskes et al, 2008). Zermann and Schmidt (1999) describe 103 patients with chronic pelvic pain whom they evaluated at a specialized neurourologic unit. They showed that a majority of the men had insufficient conscious control of their somatically innervated striated pelvic floor muscles. The patients showed various levels of identity with their pelvic floor muscles, but none was able to demonstrate the full range of pelvic floor contraction and relaxation repetitively and effortlessly. This was true whether or not there was evidence of inflammation. They conclude that their findings reflect a functional disassociation between the central nervous system and the peripheral target, the pelvic floor muscles.
Other clinicians (Anderson, 1999; Potts, 2003) believe that the source of the pain is specifically at the pelvic musculature attachment area at the sacrum, coccyx, ischial tuberosity, pubic rami, and endopelvic fascia. These areas are immediately adjacent to the prostate and bladder and can be recognized by the demonstration of a hyperirritable spot or myofascial trigger point that is painful on compression. It is hypothesized that the formation of myofascial trigger points in this area results from mechanical abnormalities in the hip and lower extremities, chronic holding patterns such as those that occur during toilet training, sexual abuse, repetitive minor trauma and constipation, sports that create chronic pelvic stimulation, trauma or unusual sexual activity, recurrent infections, and surgery (Anderson, 1999). More recently, it has been hypothesized that the pain experienced in some men with CPPS may be explained by pudendal nerve entrapment, which causes subsequent neuropathic pain (Antolak et al, 2002). Unfortunately, all these studies examining pelvic floor muscle abnormalities did not compare findings to a control group (a criticism that can apply to many of the studies cited in this etiology section).
Neuroendocrine Mechanisms
The pain associated with the chronic prostatitis syndromes is similar in many respects to neuropathic pain. Objective autonomic nervous system changes can be observed in men with a chronic prostatitis, suggesting that altered autonomic nervous system responses may be responsible for the pain associated with this chronic pelvic pain syndrome (Yilmaz et al, 2007). Pain that may have originated in the prostate or pelvic floor muscles, through mechanisms of cross-sensitization, may have spread to adjacent organs and/or structures. We are only recently beginning to understand the complexity of overlapping neuropathways and possible mechanisms underlying pelvic organ crosstalk (Malykhina, 2007).
It has been shown that men with chronic prostatitis showed evidence of dysfunctional hypothalamic-pituitary-adrenal axis function reflected in augmented awakening cortisol responses (Anderson et al, 2008). Another study evaluating the adrenocortical hormone abnormalities in men with chronic prostatitis suggested that some men with this condition may even meet the diagnostic criteria for nonclassic congenital hyperplasia (Dimitrakov et al, 2008).
Psychological Factors
Psychological factors have always been considered to play an important role in the development or exacerbation of the chronic prostatitis syndromes. Some researchers who have investigated the psychopathology in these patients concluded that this syndrome should be viewed as a psychosomatic disorder (Mendlewich et al, 1971; Mellan et al, 1973; Keltikangas-Jarvinen et al, 1982). De la Rosette and associates (1993b) compared a group of 50 patients with chronic prostatitis with a group of 50 patients seen for vasectomy and showed that, although there were significant statistical differences between the groups (with patients with chronic prostatitis scoring consistently higher personality disorder scores), these differences in scores were quite small compared with those between patients with prostatitis and psychiatric patients. Berghuis and coworkers (1996) compared 51 prostatitis patients with a group of 34 men without any chronic pain condition and concluded that depression and psychological disturbances are common among prostatitis patients. Egan and Krieger (1994) compared prostatitis patients with those seeking treatment for chronic low back pain. Major depression was more common in prostatitis patients, but back pain caused more somatically focused depression and anxiety. Ku and coworkers (2002) suggested that depression and weak masculine identity may be associated with an early stage of chronic prostatitis. A large case-control study confirmed that depression and panic disorders are significantly more common in men with chronic pelvic pain conditions than in control subjects (Clemens et al, 2008). These more recent studies demonstrate that psychological factors are involved in the disease, but it seems unjustified to label this group of patients as neurotic or as having a psychopathologic condition. However, recent analyses of the large prostatitis cohorts showed that psychological variables, such as depression, maladaptive coping techniques (e.g., pain catastrophizing and pain contingent resting), poor social support, and stress are important in chronic prostatitis outcomes (Tripp et al, 2004, 2005, 2006; Ulrich et al, 2005; Nickel et al, 2008b). Factors such as catastrophizing are particularly important because they have been found to be stronger predictors of patient pain reports than depression (Tripp et al, 2006), indicating that negative cognitive appraisals of pain experience may be a primary target for psychosocial interventions. This may be especially important given the strong association that pain catastrophizing has shown with elevations in depression, disability, and lower quality of life manifest in patients with chronic prostatitis (Tripp et al, 2005, 2006; Nickel et al, 2008b).
Association with Interstitial Cystitis (Painful Bladder Syndrome)
Interstitial cystitis, now referred to many as painful bladder syndrome or bladder pain syndrome, is an ill-defined CPPS occurring primarily in females, and a number of investigators have hypothesized that chronic nonbacterial prostatitis may have a similar cause (Pontari, 2006; Forrest et al, 2007). Unfortunately the cause of interstitial cystitis remains unknown, but the pathogenic mechanisms are theorized to be very similar to those that cause chronic prostatitis in men (Sant and Nickel, 1999; Eisenberg et al, 2003; Parsons 2003). Some researchers have proposed that in some patients diagnosed with prostatitis, a bladder-orientated interstitial cystitis mechanism actually accounts for the symptoms and that the prostate is only indirectly involved (Sant and Kominski, 1997). Certainly, the pain and voiding symptoms of interstitial cystitis and chronic prostatitis overlap to some extent (Miller et al, 1995; Novicki et al, 1998; Sant and Nickel, 1999; Forrest and Schmidt, 2004), and men with prostatitis diagnoses have findings on cystoscopic (Berger et al, 1998), urodynamic (Siroky et al, 1981), and potassium sensitivity testing (Parsons et al, 2002, 2005) very similar to those of patients with interstitial cystitis. However, Yilmaz and associates (2004) did not confirm positive potassium sensitivity testing in prostatitis patients and Keay and colleagues (2004) have shown that men diagnosed with chronic prostatitis (pain only) have normal antiproliferative factor activity whereas men diagnosed with interstitial cystitis (pain and irritative voiding symptoms) have detectable levels of urine antiproliferative factor.
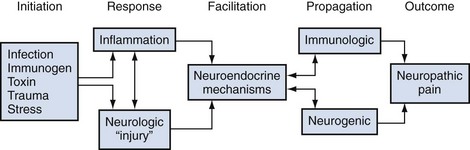
An Interrelated, Pluricausal, Multifactorial Etiology
It is likely that the nonbacterial prostatitis syndromes have a multifactorial etiology, either a spectrum of etiologic mechanisms or, more likely, a progression or cascade of events after some initiating factor. In an important review on mechanisms involved in the pathogenesis of chronic prostatitis, Pontari and Ruggieri (2004) concluded that, “the symptoms of chronic prostatitis/chronic pelvic pain syndrome appear to result from an interplay between psychological factors and dysfunction in the immune, neurological and endocrine systems.” Figure 11–2 describes a hypothetical scenario that could potentially involve most of the proposed and interrelated causes described in this section.
Definition and Classification
Traditional Classification
The traditional classification system is based on the landmark paper by Meares and Stamey (1968) describing the differential diagnosis of the prostatitis syndromes. This classic paper described in great details the serial cultures (and treatment) in four patients with chronic prostatitis and introduced the so-called Meares-Stamey four-glass test. This localization test, which segmentally assesses inflammation and cultures of the male lower urinary tract, is described later in this chapter (see Lower Urinary Tract Evaluation). Based on 10 years of clinical experience with this test, a classification system describing four categories of prostatitis was described by Drach and colleagues in 1978. Differentiation of the four categories depended on an analysis of prostatic fluid, which included microscopy (examination for white blood cells [WBCs], inflammatory cell clumps, mucus debris, oval fat bodies, and macrophages) and culturing (identifying traditional uropathogens).
Acute bacterial prostatitis was diagnosed when prostatic fluid was clinically purulent, systemic signs of infectious disease were present, and bacteria were cultured from prostatic fluid. Chronic bacterial prostatitis was diagnosed when pathogenic bacteria were recovered in significant numbers from a purulent prostatic fluid in the absence of a concomitant UTI or significant systemic signs. Nonbacterial prostatitis was diagnosed when significant numbers of bacteria could not be cultured from prostatic fluid but the fluid consistently revealed microscopic purulence. Prostatodynia was diagnosed in the remaining patients who had persistent pain and voiding complaints as in the previous two categories but who had no significant bacteria or purulence in the prostatic fluid. This clinical differentiation of the prostatitis syndromes is now referred to as the “traditional” classification system (Table 11–1).
Table 11–1 Classification System for the Prostatitis Syndromes
| TRADITIONAL | NATIONAL INSTITUTES OF HEALTH | DESCRIPTION |
|---|---|---|
| Acute bacterial prostatitis | Category I | Acute infection of the prostate gland |
| Chronic bacterial prostatitis | Category II | Chronic infection of the prostate gland |
| N/A | Category III chronic pelvic pain syndrome (CPPS) | Chronic genitourinary pain in the absence of uropathogenic bacteria localized to the prostate gland employing standard methodology |
| Nonbacterial prostatitis | Category IIIA (inflammatory CPPS) | Significant number of white blood cells in expressed prostatic secretions, post–prostatic massage urine sediment (VB3) or semen |
| Prostatodynia | Category IIIB (noninflammatory CPPS) | Insignificant number of white blood cells in expressed prostatic secretions, post–prostatic massage urine sediment (VB3) or semen |
| N/A | Asymptomatic inflammatory prostatitis (AIP) | White blood cells (and/or bacteria) in expressed prostatic secretions, post–prostatic massage urine sediment (VB3), semen or histologic specimens of prostate gland |
The significant limitations of this classification system included the abandonment by most physicians of the rigorous Meares-Stamey four-glass test (Moon, 1997; Nickel et al, 1998a; McNaughton Collins et al, 1999, 2000a), the realization that only the very rare patient would be subsequently diagnosed with chronic bacterial prostatitis, the perception that patients responded to specific therapy (e.g., antibiotics) independent of this classification system, and the dawning realization that, in many cases, patients presenting with prostatitis syndromes were not easily classified into one of these categories (e.g., patients in whom specific cultures were negative because of previous antibiotic therapy or those thought to have a cause other than the prostate gland) (Nickel, 1998a).
National Institutes of Health Classification
The limitations of the traditional diagnostic algorithm and traditional classification system led to the development of the National Institutes of Health (NIH) classification system (see Table 11–1) (Krieger et al, 1999). The new definition recognized that pain is the main symptom in “abacterial chronic prostatitis” (with variable voiding and sexual dysfunction), and it was the optimal criterion to differentiate chronic prostatitis patients from control patients or patients experiencing other genitourinary problems (e.g., BPH). This classification differed from the traditional system in two main areas: the descriptions of category III CP/CPPS and category IV asymptomatic inflammatory prostatitis.
Category I is identical to the acute bacterial prostatitis category of the traditional classification system. Category II is identical to the traditional chronic bacterial prostatitis classification, except that it now usually refers to patients with recurrent lower urinary tract infections (with a prostate nidus of infection) (Schaeffer, 2006). Category III is defined as the “presence of genitourinary pain in the absence of uropathogenic bacteria detected by standard microbiologic methodology.” This syndrome is further categorized into category IIIA,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree