The BED normally carries a subscript corresponding to the assumed α/β since it is clearly different for large and small α/β. Thus, for any given fractionation schedule, one must determine BED for both early (including tumor) and late effects.
Two alternatives to a standard (1.8–2 Gy) fractionation schedule are known as hyperfractionation and hypofractionation. In a hyperfractionated schedule, smaller than standard fraction sizes are used, usually to a higher total dose, so that a higher rate of tumor control might be achieved for a fixed rate of late complications. In contrast, with hypofractionation, relatively large fractions are used to a lower total dose. The main benefit of such a schedule is a shorter overall course of treatment, but the risk of long-term complications may be relatively higher. In palliative cases, it often makes sense to expedite treatment and late effects may be less important in patients with a limited prognosis.
Interestingly, there is also some evidence that a hypofractionated schedule may also be beneficial in the definitive treatment of prostate cancer. This speculation arises from studies of dose and tumor control following treatment of prostate cancer with either external radiation or brachytherapy. Because different brachytherapy sources emit radiation at different rates, it is possible to get rough estimates of the α/β for prostate cancer; and some studies suggest that prostate cancer may have, among common human neoplasms, a uniquely low α/β of ~1.5. If these estimates are accurate, then the therapeutic ratio for prostate cancer relative to late effects may, in fact, be optimized by using larger than standard fractions. Trials of hypofractionation are in progress, but until they are complete, the standard of care is still to use the standard fractions (1.8–2 Gy) [4].
New Technologies and Equipment
Three Dimensional Conformal Radiotherapy and Intensity Modulated Radiotherapy (3DCRT and IMRT)
Along with innovations in computing and imaging, radiotherapy techniques have improved. Powerful workstations facilitate the examination of multiple treatment strategies and allow radiation oncologists to optimize the ratio of dose delivered to the tumor target and minimize the dose absorbed by uninvolved normal structures [5].
3DCRT is defined as treatment using a volumetric imaging dataset, such as CT or MRI, and segmentation of that dataset into relevant structures, usually tumor targets (i.e. prostate and seminal vesicles) and normal structures (i.e. rectum and bladder). Multiple beams are selected by the oncologist based upon separation of tumor from normal and the beams are each sharply collimated with movable lead leaves (multileaf collimation). The multiple beams (commonly 4–6 for prostate cancer) intersect at the target resulting in a high dose and uniform dose delivery. For 3DCRT, each beam is relatively “flat”, that is the dose is uniform as one traverses the beam aperture. The dose received by normal tissues is also calculated and can be displayed volumetrically using a dose-volume histogram, which shows the percent of the structure that receives above a certain dose. The dose-volume histogram can be analyzed to predict the risk of toxicity, since toxicity is related the dose received by a certain volume of an organ. For example, many prostate cancer treatments are designed to treat the prostate fully to up to 78 Gy, but to limit the rectum such that no more than 25 % of the rectum receives more than 70 Gy.
IMRT is a more recent extension of the 3DCRT principles. However, as implied by the name, the intensity of each beam is non-uniform across the aperture. This allows even more conformal dose distributions and greatly increases the therapeutic ratio of external beam radiotherapy. Figure 14.1 illustrates schematically the variation in intensity across an IMRT aperture and illustrates the improvement in conformality that can be achieved with IMRT versus 3DCRT for prostate cancer.
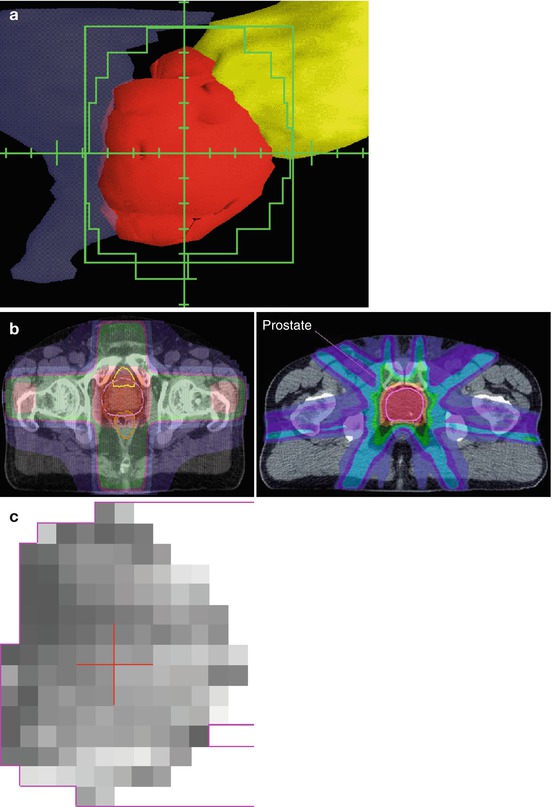

Fig. 14.1
(a) Beam’s-eye-view display showing prostate (red) surrounded by custom aperture (green) which avoids rectum (blue) and bladder (yellow). (b) 3DCRT dose distribution (left) and IMRT dose distribution (right). The prostate target is outlined in pink and the high dose region is in red. Note the uniform intensity across the beam in the 3DCRT plan versus the modulated intensity across the beams in the IMRT plan. (c) Gray scale view if the intensity map of a single prostate IMRT beam showing the variation in intensity that, when combined with multiple similarly modulated beams, results in the highly conformal treatment seen in b
As mentioned above, ionizing radiotherapy can be delivered with particles as well as with photons or x-rays. Electrons are commonly employed to treat superficial targets as these light particles have little ability to penetrate deeply into tissue. High energy protons, which weigh 2,000 times more than an electron, are capable of penetrating deeply into tissue. Because protons are charged particles, they deposit their energy via electromagnetic interaction with the charged particles in tissue (electrons and nuclei). Interestingly, as the proton begins to give up its energy and slow down, this increases its energy deposition, which makes it slow down even more quickly, increasing further the energy deposition, and so it stops quite abruptly.
This leads to a relatively large about of ionization and subsequent DNA strand breaks near the end of the protons range and this is called the Bragg peak. This peak in ionization is favorable for radiation planning and dose delivery and has led to the proliferation of somewhat expensive proton treatment facilities at selected locations world-wide. Clinical trials will be required to determine whether proton beam treatment is more favorable than the widely available IMRT techniques.
Daily Localization
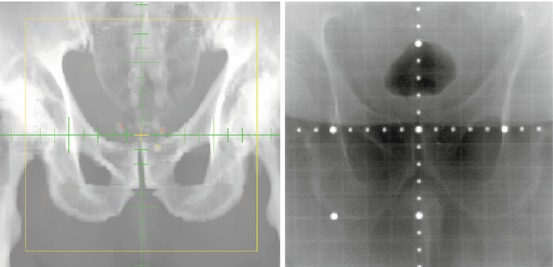
As radiotherapy techniques (IMRT in particular) result in more focused radiation doses, there is more concern about accuracy in targeting. After all, the careful planning that goes into and IMRT treatment plan is only put to good use if the patient is treated in the same position as they were in during the imaging dataset used in the planning process. Traditionally this was done by obtaining bone images during treatment. However, if the target is moveable with respect to the skeleton (as the prostate can be, depending upon bladder and rectal filling), bone imaging may be inadequate. One strategy for daily location verification is to implant fiducial markers into the target organ, imaging these radio-opaque markers prior to therapy, and then correcting the patient’s position, if necessary [6]. (Fig. 14.2) Other strategies use daily pretreatment ultrasound [7] or, for pelvic tumors, inflated rectal balloons [8].
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







