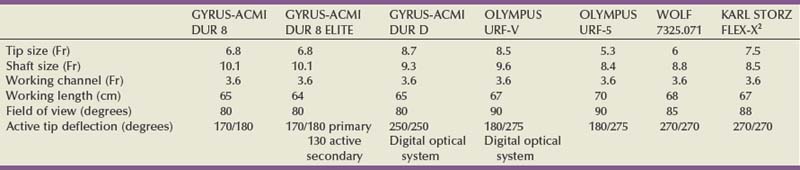
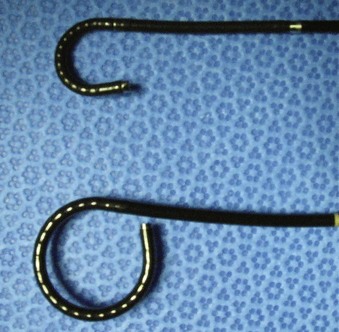
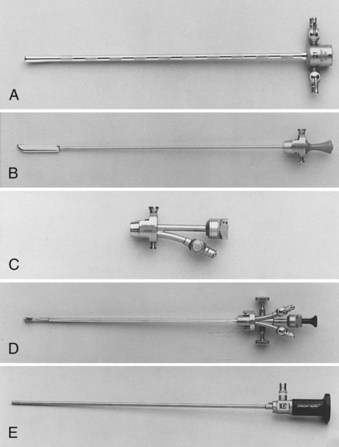
Branden Duffey, DO, Manoj Monga, MD, FACS Retrograde visualization of the urinary tract is a routine part of the urologist’s practice and may be performed in a variety of settings. Straightforward procedures (i.e., cystoscopy with minimal intervention) may be performed in the office setting and requires only an endoscope, light source, and irrigating fluid. The urologist views the procedure through the optical eyepiece at the proximal end of the instrument. Either xenon or halogen external light sources deliver cool light to the endoscope through a fiberoptic cable. Typical irrigation fluids include sterile water, glycine, or normal saline. If electrocautery use is anticipated, a solution free of electrolytes should be used. Video-endoscopic units, comprising a light source, camera for the endoscope, image processor and recorder, and monitor, are usually arranged on a mobile tower and commonly found in both the office and operating room settings (Fig. 8–1). Images are transmitted to the image processor by a camera attached to the eyepiece and displayed on a viewing monitor. In the case of digital endoscopes there is no eyepiece and the image is sent directly to the image processor. In both cases, still images and live sequences can be transferred to a recording device for incorporation into the medical record, postprocedure review, and patient education. Video-endoscopic units are advantageous in that they (1) avoid surgeon contact with bodily fluids; (2) may facilitate more ergonomic surgeon positioning during the procedure; (3) enhance the teaching of endourologic skills; and (4) may decrease discomfort in male patients during cystoscopy performed using local anesthesia (Patel et al, 2007). Light source modifications, specifically narrow band imaging and porphyrin-based fluorescence, have been developed in efforts to improve the detection of urothelial carcinoma during endoscopy. Narrow band imaging technology filters white light into two separate bands (415 nm and 540 nm) that are absorbed by hemoglobin, which aids in the detection of hypervascular urothelial neoplasia (Herr and Donat, 2008). In fluorescence-based endoscopy, a solution of 5-aminolevulinic acid or hexaminolevulinate is instilled in the bladder and then inspected with blue light (wavelength 380 to 450 nm). These substances aid in tumor detection because they are converted to porphyrins that preferentially accumulate in neoplastic cells and fluoresce red when illuminated with blue light (Kriegmair et al, 1996). Urology-specific endoscopy suites are found in the operating room and generally have full video capabilities in addition to fluoroscopy provided by either a mobile C-arm or endoscopy table. Fluoroscopy is used to perform retrograde pyelography and ureteral stenting and to localize endoscopes and instruments during the procedure. It is imperative that operating room personnel minimize radiation exposure by wearing leaded aprons, thyroid shields, and eyewear. Rigid cystourethroscopes require assembly before use and are composed of a sheath, obturator, bridge, and optical telescopic lens (Fig. 8–2). Markings on the sheath indicate the endoscope size as well as the maximum size of a single instrument or two instruments (as in the case of a bridge with two working channels) the endoscope will accept (Fig. 8–3). Irrigant is instilled through tubing that is attached to the sheath. Obturators are inserted into the working sheath to provide a smooth blunt surface during endoscope passage. Typically, an obturator will be employed for insertion of the rigid cystoscope in females whereas direct visualization will be utilized for cystoscope insertion in males. The bridge is attached to the sheath and may have one or two working channels, facilitating the passage of instruments into the endoscope. The Albarran bridge enables the urologist to deflect catheters and wires placed through the working channel by manipulating a lever attached to the bridge. The telescopic lens is attached to the sheath by means of the bridge. The light source cable is connected to the telescope. (A to E, Courtesy of Circon Corp., Santa Barbara, CA.) Flexible cystourethroscopes are available in fiberoptic and digital models (Fig. 8–4). Traditional flexible endoscopes contain fiberoptic bundles within a flexible shaft that illuminate the viewing area and transmit images to the eyepiece. The tip of the endoscope may be deflected up to 210 degrees by a lever near the eye piece. The endoscope shaft has a channel that is shared for irrigation and the passage of working instruments. Recent advances in solid-state camera chips have facilitated the development of digital cystourethroscopes. In these models an imaging chip is placed at the distal end of the endoscope that converts light into an electrical charge that is eventually displayed on a viewing screen. Digital systems are advantageous because they have higher optical resolution, contrast, and color differentiation; in many centers digital systems have replaced fragile fiberoptic systems, which are prone to breakage (Quayle et al, 2005; Borin et al, 2006). However, the durability of digital scopes has yet to be established. Before performing cystourethroscopy it is important to ensure the patient does not have a urinary tract infection, because mechanical manipulation and bladder distention can exacerbate the infection. Informed consent should be obtained after explaining the procedure, its purpose, and the associated risks and benefits. Antimicrobial prophylaxis is not recommended before simple cystourethroscopy unless patient risk factors are present (Table 8–1) (Wolf et al, 2008). When risk factors are present or additional manipulation (e.g., bladder biopsy, ureteral catheterization) is anticipated, prophylactic antibiotics should be administered before the procedure with an expected treatment duration of 24 hours or less. Antimicrobial agents of choice in these situations are the fluoroquinolones and trimethoprim-sulfamethoxazole. The patient is placed in the dorsal lithotomy position for rigid cystourethroscopy or the supine position for flexible cystourethroscopy (with a slight frog-leg position for females) and prepped and draped sterilely. Table 8–1 Patient Risk Factors Precluding Simple Cystoscopy Without Antimicrobial Prophylaxis Preoperative intraurethral injection of a water-soluble lubricant-anesthetic is often used in an attempt to provide greater patient comfort and enable the surgeon to access the upper urinary tract and perform bladder and urethral biopsies as well as bladder fulguration. In male patients a urethral clamp may be used to retain the urethral anesthetic, allowing for prolonged direct contact with the urethral mucosal surfaces before instrumentation. Goldfischer and colleagues (1997) found that 30 mL of intraurethral lidocaine gel left to dwell for 20 minutes before rigid cystoscopy significantly reduced procedure-related pain in men but not women. Most randomized prospective placebo-controlled studies of flexible cystoscopy conclude that lubricant-anesthetic instillation does not decrease patient discomfort (Patel et al, 2008b). Several strategies have been evaluated to improve patient comfort and tolerance during cystourethroscopy. Positioning the video monitor so the patient and surgeon can simultaneously view the procedure may be beneficial in male patients but not females (Patel et al, 2007, 2008a). A real-time explanation of the procedure may decrease patient anxiety and discomfort. Specifically, male patients are reassured and counseled that discomfort will be encountered in the membranous and prostatic urethra; they are informed that the length of this segment is 2 to 5 cm, and they are encouraged to relax their pelvic muscles during this portion of the examination to facilitate scope passage (Taghizadeh et al, 2006). In males the glans is grasped and the penis held on stretch at 90-degree angle to the abdominal wall during visualization of the fossa navicularis, penile urethra, and bulbar urethra. For flexible cystoscopy, the glans is held on stretch between the third and fourth digits, allowing the thumb and forefinger to remain free to guide the endoscope through the urethra (Fig. 8–5). The cystourethroscope tip is inserted and the irrigant turned on, distending the urethra to allow for clear visualization. Periurethral glands of Littre are noted as they enter the urethra dorsally. Strictures may be encountered that require dilation (see Chapter 7) or visual internal urethrotomy (see Chapter 36). The membranous urethra is identified by its striated mucosa and may visibly contract. As the membranous urethra is traversed, the cystourethroscope must be directed anteriorly to navigate the transition from the proximal bulbar urethra to the membranous urethra and into the prostatic urethra. As the distal prostatic urethra is entered, the verumontanum is identified posteriorly. Prostate length, the lateral lobes, and the median lobe are evaluated as the cystourethroscope continues its passage to the bladder. Additional anterior angulation may be necessary to elevate the endoscope over the bladder neck and pass into the bladder. Patients with urinary drainage from a suprapubic catheter deserve special mention based on the additional clinical scenarios and technical aspects associated with evaluation of the lower urinary tract. Indications for cystourethroscopy are the same as described earlier but also include regular inspection for bladder stones and surveillance for bladder tumors in patients with suprapubic catheters longer than 5 to 10 years (Kaufman et al, 1977; DeVivo et al, 1985; Hess et al, 2003; Subramonian et al, 2004). Endoscopy may be performed per urethra (when patent) or through the suprapubic tract. Navigation and visualization of the suprapubic tract is usually straightforward in patients with normal body habitus and a mature urinary tract. Conversely, immature urinary tracts or those traversing a large mobile pannus may be difficult to follow. When these situations occur, it is helpful to place a wire through the suprapubic tract into the urinary bladder to guide the endoscope during inspection. The wire can be backloaded into the working channel to further guide the endoscope. Rigid cystoscopes may fit into large mature urinary tracts and are advantageous when ancillary procedures are indicated, but they may be difficult to maneuver in long urinary tracts and orientation in the bladder can be challenging. Flexible cystoscopes offer improved maneuverability and may fit into smaller urinary tracts. For prolonged endoscopic procedures, continuous irrigation can be facilitated by endoscopy through the urinary tract with simultaneous drainage of irrigation through the urethra or vice versa. Identification of the ureteral orifices may be challenging owing to edema related to the indwelling catheter or to the angle of vision through the tract. This may be overcome by the use of a flexible cystoscope to visualize the efflux of urine after administration of intravenous indigo carmine or methylene blue. Continent urinary diversion is a common urinary reconstructive technique used after cystectomy. Diversions can be broadly categorized as cutaneous continent diversions or orthotopic diversions. In cutaneous continent urinary diversions, detubularized bowel is arranged to create a urinary reservoir with a catheterizable limb anastomosed to the umbilicus or another site on the abdominal wall that provides access for drainage. In orthotopic diversions the urinary reservoir is anastomosed to the urethra, allowing for volitional voiding. Detailed understanding of the diversion construction is crucial before any endoscopic intervention. Key elements include the type and location of the ureteroenteric anastomosis, the presence or absence of an afferent limb, and the continence mechanism employed. Many neobladders have an afferent limb extending from the reservoir to which the ureters are anastomosed. In orthotopic neobladders the continence mechanism is the external urinary sphincter. Typical continence mechanisms in cutaneous catheterizable diversions include the tunneled appendix (Mitrofanoff), the tapered/imbricated terminal ileum and ileocecal valve, and the intussuscepted nipple valve (see Chapter 86). The Mitrofanoff and tapered/imbricated ileal continence mechanisms are fragile, and aggressive manipulation can lead to stomal stenosis and/or loss of urinary continence. In these cases, simple visualization and reservoir filling through the catheterizable channel may be performed with small caliber flexible endoscopes (e.g., a flexible ureteroscope), but if ancillary procedures are indicated, percutaneous access should be used (Patel and Bellman, 1995). Similarly, percutaneous access for interventions other than simple diagnostic procedures in orthotopic neobladders may avoid the risk of bladder neck contracture or sphincteric damage. In both types of diversions, orientation and visualization can be impaired by mucus and debris, bowel peristalsis, mucosal folding, and tortuous afferent limbs. Steps to overcome these difficulties include thorough irrigation and use of fluoroscopy with intravesical administration of a contrast agent to identify the os of the afferent limb and guide endoscope advancement. When present, afferent limbs are best visualized with flexible endoscopes because they are best suited to navigate limb folding, kinking, and tortuosity. Key Points Cystourethroscopy Ureteroscopy is a standard urologic technique that provides direct visualization of the upper urinary tract, facilitating both diagnostic and therapeutic interventions. Ureteroscopy is most commonly used for the treatment of nephrolithiasis (see Chapter 48). Other common indications include evaluation of lateralizing hematuria, abnormal urinary cytology, and abnormal upper tract imaging studies. Surveillance and treatment of upper tract urothelial carcinoma may be performed using ureteroscopic techniques (see Chapter 53). Ureteroscopy may also be utilized in cases of ureteral stricture and ureteropelvic junction obstruction (see Chapter 41). Table 8–2 lists some of the currently available flexible ureteroscopes. The distal tip of these endoscopes ranges from 5.3 to 8.7 Fr and gradually tapers toward the proximal shaft. Active deflection of the distal tip typically ranges from 130 to 250 degrees in one direction and 160 to 275 degrees in the other, with newer flexible ureteroscopes having exaggerated primary deflection that allows for improved visualization of the lower pole and increased ease of manipulation (Fig. 8–6) (Monga et al, 2007). Secondary active deflection, available in the DUR-8 Elite (Gyrus-ACMI, Southborough, MA) and the Stryker Flexvision (Stryker Endoscopy, San Jose, CA), refers to a segment of active deflection several centimeters proximal to the area of primary deflection that, when activated by a second hand-piece lever, provides an additional 130 degrees of visualization and facilitates entry into the lower pole calyx. Passage of working instruments (e.g., lasers, baskets) tends to increase the stiffness of the ureteroscope shaft, reduce maximal deflection, and decrease irrigation flow through the shared 3.6-Fr channel (Abdelshehid et al, 2005; Bach et al, 2008; Paffen et al, 2008). Imaging systems in flexible ureteroscopes are either traditional fiberoptic systems or digital, based on CCD or complementary metal–oxide–semiconductor (CMOS) technology. Preliminary clinical experience suggests that digital ureteroscopes offer improved optical resolution compared with their fiberoptic counterparts (Traxer et al, 2007). Technologic advancements in flexible ureteroscopes have enabled reductions in shaft diameter, improved visualization, and provided a greater range of deflection, but they remain fragile instruments with significant purchase and repair costs (Chiu et al, 2004; Sung et al, 2005; Monga et al, 2006). Guidewires used during retrograde instrumentation serve to provide access to a particular area of the urinary tract and serve as a guide to pass catheters, stents, and sheaths (Clayman et al, 2004; Liguori et al, 2008
Equipment
Cystourethroscopy
Equipment
Patient Preparation
Technique
Special Considerations
Suprapubic Tubes
Continent Urinary Diversions
Upper Urinary Tract Endoscopy
Indications
Equipment
Ureteroscopes
Ancillary Equipment
Wires
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree